In the name of God RAPIDACTING INSULINS F

































- Slides: 59

In the name of God

RAPID-ACTING INSULINS F. Sarvghadi MD Associate professor of endocrinology. Research institute for endocrine sciences Shahid Beheshti University Of Medical Sciences

AGENDA • • • Introduction Pharmacokinetic Properties of Insulin Molecular Structure & Dosage Forms Lispro Glulisine Aspart Comparison between Insulin Regular & Rapid-Acting Insulin in DM 1(Hb. A 1 C Reduction, rate of Hypoglycemia , Quality of Life) Comparison between Insulin Regular & Rapid-Acting Insulin in DM 2(Hb. A 1 C Reduction , rate of Hypoglycemia , Quality of Life) Rapid –Acting Insulin in Pregnancy Rapid –Acting Insulin in Elderly Conclusion

Introduction • Insulin has been commercially available since the early 1920 s and is still the mainstay of therapy for most people with DM worldwide. • Currently, almost all insulin used worldwide is of recombinant human origin or an analogue. Hirsch IB. Insulin analogues. NEJM 2005

Different kind of Insulin preparation

What are insulin analogues? • The need for production of insulin analogues resulted from the fact that the pharmacokinetics of the available insulins did not sufficiently match the physiologic secretion of insulin, both during fasting as well as postprandially. • Rapid acting insulin analogues are peptides that result from the transformation of the insulin molecule through the addition or exchange of certain amino-acids. These transformations give the insulin molecule certain desirable characteristics concerning the speed and stability of its absorption. Davidson, M. B. Diabetes Care 2005 -28: 494– 5

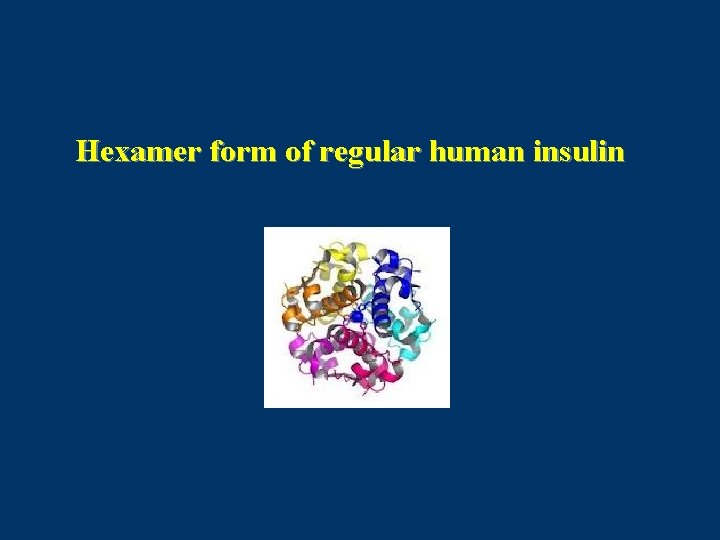
Regular Human Insulin • After SQ injection regular insulin tends to dissociate from its normal hexameric form, first into dimers and then monomers. • Only dimeric & monomeric forms can pass through the endothelium into the circulation. • The resulting delay in the onset and duration of action, limits effectiveness in controlling postprandial glucose. • Dose dependent pharmacokinetics: with prolonged onset, peak, duration of action with higher doses. Med Clin North Am 1998

Hexamer form of regular human insulin

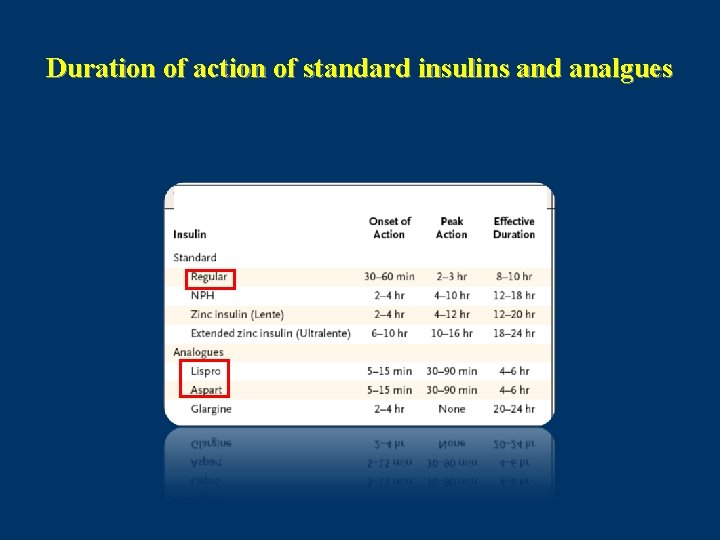
Pharmacokinetic Properties of Insulin Preparation Short Acting Insulin(Regular) • Onset of action in 0. 5 -1 hour – • Peak activity: 2 -4 hours Duration of activity : 6 -8 hours Med Clin North Am 1998

Pharmacokinetic Properties of Insulin Preparation Rapid acting insulin analogues: • Onset of action in 15 minutes • Peak activity: 1 – 1. 5 hour • Duration of activity : 3 -4 hours Lancet 1997 Jan

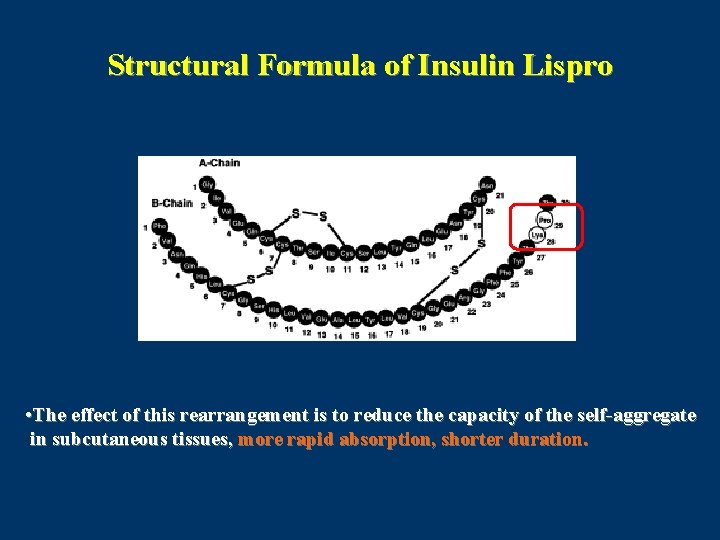
Insulin Lispro (Humalog) • Recombinant DNA origin. • Amino acids at positions 28 and 29 on the human insulin B chain are reversed. Lys(B 28), Pro(B 29) insulin. • Insulin lispro, the first rapidly acting analogue that was developed, differs from regular insulin by virtue of its capacity to dissociate rapidly into monomers in subcutaneous tissue. • Lispro is created in a special nonpathogenic laboratory strain of Ecoli that has been genetically altered by the addition of the gene for insulin lispro. Diabetes 2004

Structural Formula of Insulin Lispro • The effect of this rearrangement is to reduce the capacity of the self-aggregate in subcutaneous tissues, more rapid absorption, shorter duration.

Insulin Lispro • Dosage forms: 100 units per ml (3 ml) Prefilled cartridge Prefilled disposable pen • Administration : Within 15 minutes before or immediately after a meal.

Insulin Aspart (Novorapid) • The second rapidly acting analogue that was introduced was insulin aspart. With its proline having been replaced by the negatively charged aspartic acid, this analogue has an insulinreceptor affinity similar to that of human insulin • Substitution of aspartic acid for proline in position B 28. • Dosage forms: 100 units /ml(3 ml) Prefilled disposable pen.

Structural Formula of Insulin Aspart

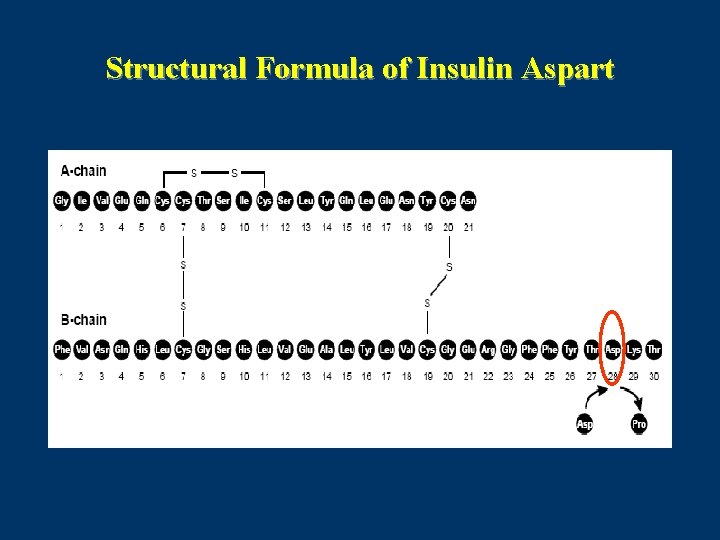
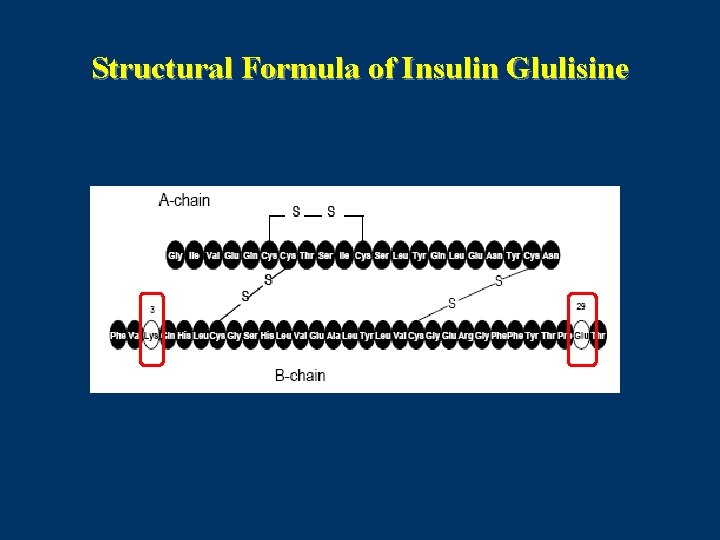
Insulin Glulisine (Apidra) • Insulin glulisine involves substitution of the asparagine at position B 3 with lysine and the lysine in position B 29 with glutamic acid. • Dosage forms: 100 units ⁄ml (3 ml cartridge , 10 ml vial)

Structural Formula of Insulin Glulisine

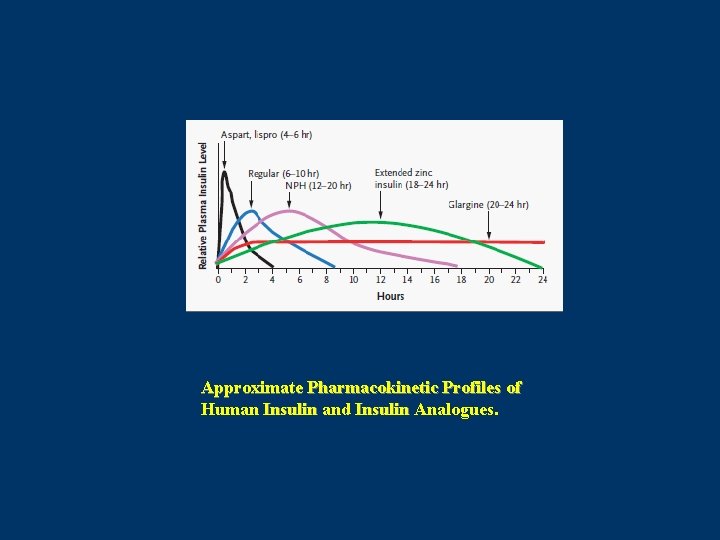
Pharmacokinetic Properties of Insulin Analogues. In general, injection of these rapidly acting analogues results in twice the maximal concentration and takes about half the time to reach the maximal concentration as do equivalent doses of regular insulin. Peak insulin action occurs approximately twice as fast with the analogues as with regular insulin. Use of these rapidly acting analogues also results in less variability in absorption at the injection site and possibly in less variation between and within patients. Howey DC. Diabetes 1994

Approximate Pharmacokinetic Profiles of Human Insulin and Insulin Analogues.

Duration of action of standard insulins and analgues

• Use of these rapidly acting analogues also results in less immunologic reactions. • Another important consideration in regard to rapidly acting analogues is that less snacking is required with their use. • Less postabsorptive hypoglycemia. Hirsch IB. Insulin analogues. NEJM 2005

Short-acting insulin analogues vs. regular human insulin

Comparison of Lispro Insulin with Regular Insulin in DM 1 • The postprandial rise was reduced at 1 h by 23 mg/dl and at 2 h by 36 mg/dl in patients treated with insulin lispro (P < 0. 001). • The rate of hypoglycemia was 12% less with insulin lispro (P < 0. 001) • The number of hypoglycemic episodes was less with insulin lispro (P < 0. 001). • The largest relative improvement was observed at night. Anderson Jr; Diabetes 1997

Conclusion Insulin lispro compared with regular human insulin, in IDDM patients: Improves postprandial control, Improves patient convenience, Reduces hypoglycemic episodes. Anderson Jr; Diabetes 1997

Efficacy and safety of insulin glulisine, in the treatment of diabetes mellitus • Several studies indicated a statistically significant decrease of hemoglobin A 1 C (A 1 C) with glulisine compared with regular insulin (0. 10 decrease). • No difference in A 1 C control was found compared with insulin aspart or lispro. Ann Pharmacother. 2009 Apr; 43(4): 658 -68

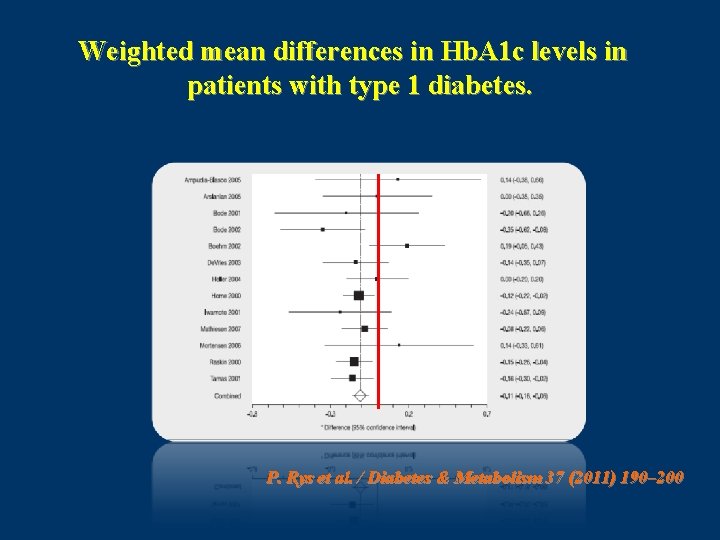
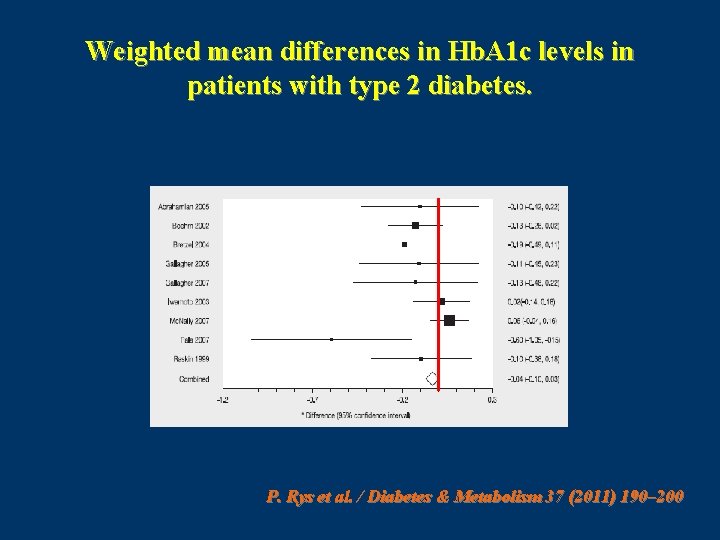
This meta-analysis was based on the results of randomized controlled trials (RCTs) that enrolled patients with either T 1 DM or T 2 DM and with no restrictions on age. ( N = 27 ) P. Rys et al. / Diabetes & Metabolism 37 (2011) 190– 200

Weighted mean differences in Hb. A 1 c levels in patients with type 1 diabetes. P. Rys et al. / Diabetes & Metabolism 37 (2011) 190– 200

Weighted mean differences in Hb. A 1 c levels in patients with type 2 diabetes. P. Rys et al. / Diabetes & Metabolism 37 (2011) 190– 200

* * * * P. Rys et al. / Diabetes & Metabolism 37 (2011) 190– 200

Conclusion • systematic review of the literature show that, in T 1 DM patients, treatment with IAsp resulted in moderately better metabolic control, as demonstrated by reductions in Hb. A 1 c and PPG, and no change in FG. • It was also established that using IAsp instead of RHI resulted in greater treatment satisfaction and a significant reduction of risk for nocturnal hypoglycaemic episodes, but not severe hypoglycemia. In T 2 DM patients, the present meta-analysis showed some improvement in PPG, but not for the other outcomes. P. Rys et al. / Diabetes & Metabolism 37 (2011) 190– 200

Include 68 RCT for rapid acting analogues up to April 2007

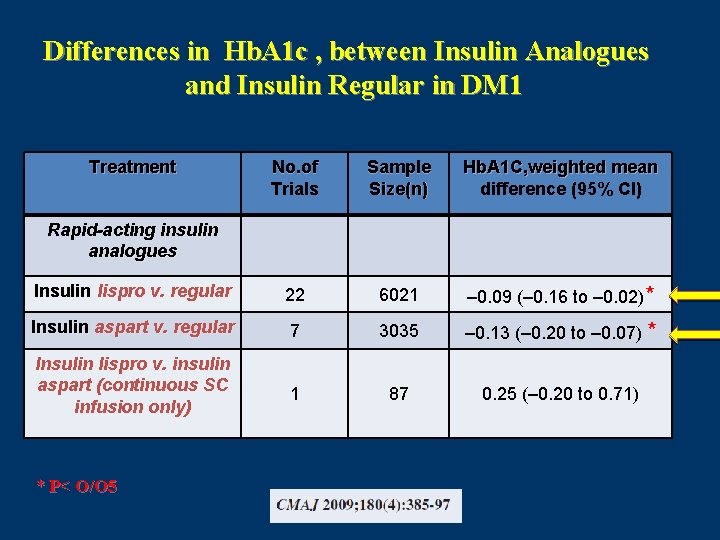
Differences in Hb. A 1 c , between Insulin Analogues and Insulin Regular in DM 1 Treatment No. of Trials Sample Size(n) Hb. A 1 C, weighted mean difference (95% CI) Insulin lispro v. regular 22 6021 – 0. 09 (– 0. 16 to – 0. 02)* Insulin aspart v. regular 7 3035 – 0. 13 (– 0. 20 to – 0. 07) * Insulin lispro v. insulin aspart (continuous SC infusion only) 1 87 0. 25 (– 0. 20 to 0. 71) Rapid-acting insulin analogues * P< O/O 5

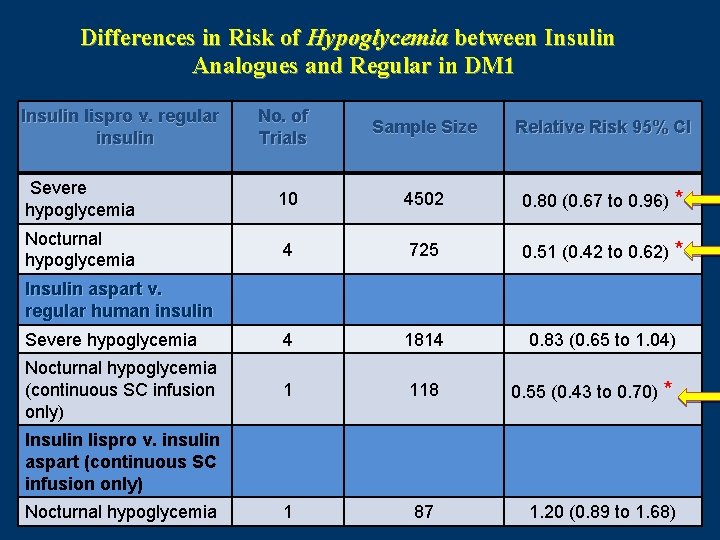
Differences in Risk of Hypoglycemia between Insulin Analogues and Regular in DM 1 Insulin lispro v. regular insulin No. of Trials Sample Size Relative Risk 95% CI Severe hypoglycemia 10 4502 0. 80 (0. 67 to 0. 96) * Nocturnal hypoglycemia 4 725 0. 51 (0. 42 to 0. 62) * Severe hypoglycemia 4 1814 0. 83 (0. 65 to 1. 04) Nocturnal hypoglycemia (continuous SC infusion only) 1 118 0. 55 (0. 43 to 0. 70) * 1 87 1. 20 (0. 89 to 1. 68) Insulin aspart v. regular human insulin Insulin lispro v. insulin aspart (continuous SC infusion only) Nocturnal hypoglycemia

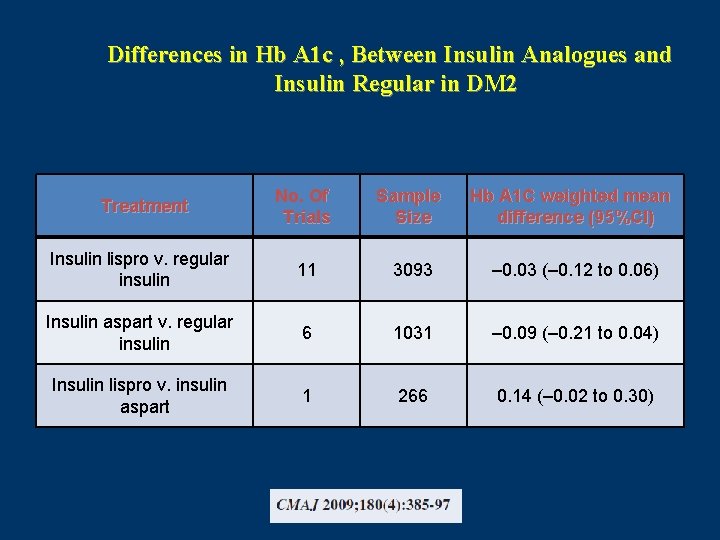
Differences in Hb A 1 c , Between Insulin Analogues and Insulin Regular in DM 2 No. Of Trials Sample Size Hb A 1 C weighted mean difference (95%CI) Insulin lispro v. regular insulin 11 3093 – 0. 03 (– 0. 12 to 0. 06) Insulin aspart v. regular insulin 6 1031 – 0. 09 (– 0. 21 to 0. 04) Insulin lispro v. insulin aspart 1 266 0. 14 (– 0. 02 to 0. 30) Treatment

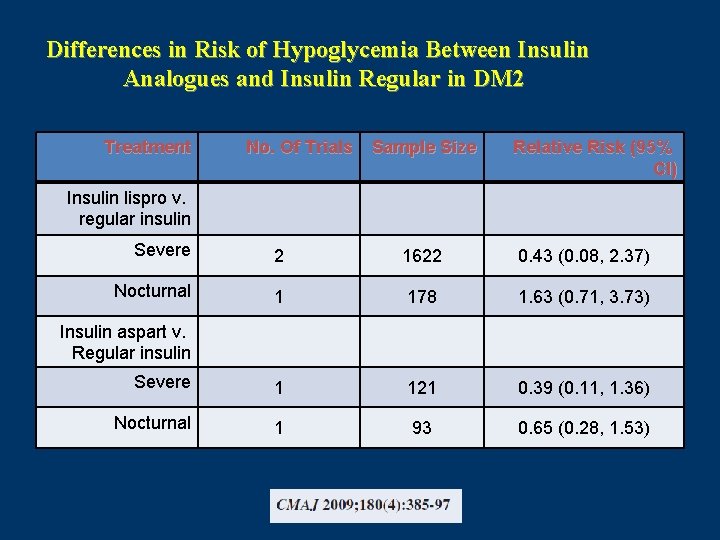
Differences in Risk of Hypoglycemia Between Insulin Analogues and Insulin Regular in DM 2 Treatment No. Of Trials Sample Size Relative Risk (95% CI) Insulin lispro v. regular insulin Severe 2 1622 0. 43 (0. 08, 2. 37) Nocturnal 1 178 1. 63 (0. 71, 3. 73) Severe 1 121 0. 39 (0. 11, 1. 36) Nocturnal 1 93 0. 65 (0. 28, 1. 53) Insulin aspart v. Regular insulin

Quality of Life in DM 1 • Patients generally preferred rapid-acting insulin analogues over regular insulin because of flexibility in dosing relative to mealtimes. • Some studies that assessed quality of life and patient satisfaction reported statistically significant improvements with the use of rapid –acting insulin compared with regular insulin.

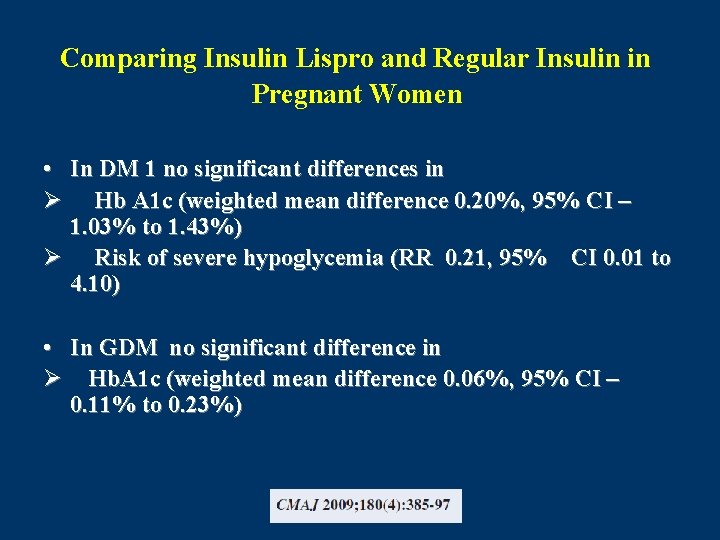
Comparing Insulin Lispro and Regular Insulin in Pregnant Women • In DM 1 no significant differences in Ø Hb A 1 c (weighted mean difference 0. 20%, 95% CI – 1. 03% to 1. 43%) Ø Risk of severe hypoglycemia (RR 0. 21, 95% CI 0. 01 to 4. 10) • In GDM no significant difference in Ø Hb. A 1 c (weighted mean difference 0. 06%, 95% CI – 0. 11% to 0. 23%)

Comparing Insulin Aspart with Regular Insulin in Pregnant Women with DM 1 • No significant differences in ; Ø Hb. A 1 c (weighted mean difference – 0. 08%, 95% CI – 0. 28% to 0. 12%) Ø Risk of severe hypoglycemia (RR 1. 14, 95% CI 0. 76 to 1. 71) Ø Risk of overall hypoglycemia (RR 1. 04, 95% CI 0. 98 to 1. 11)

Aspart in Pregnancy The comparison of insulin aspart (IAsp) with human insulin (HI) for fetal and perinatal outcomes of type 1 diabetes in pregnancy. Subjects were pregnant (gestational age; <10 weeks) 322 women with type 1 diabetes received IAsp (n = 157) or HI (n = 165). Moshe Hod Am J Obstet Gynecol. 2008 Feb

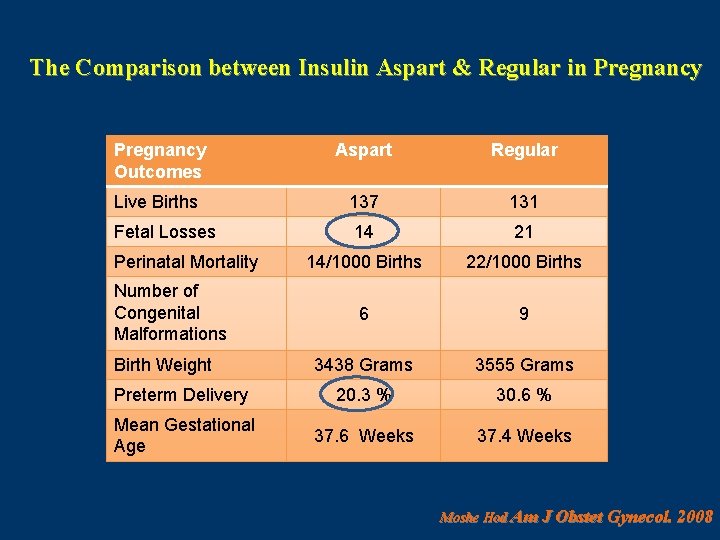
The Comparison between Insulin Aspart & Regular in Pregnancy Outcomes Aspart Regular Live Births 137 131 Fetal Losses 14 21 14/1000 Births 22/1000 Births 6 9 3438 Grams 3555 Grams Preterm Delivery 20. 3 % 30. 6 % Mean Gestational Age 37. 6 Weeks 37. 4 Weeks Perinatal Mortality Number of Congenital Malformations Birth Weight Moshe Hod Am J Obstet Gynecol. 2008

CONCLUSION The fetal outcome using aspart insulin was comparable with regular insulin with a tendency toward fewer fetal losses and preterm deliveries. Moshe Hod Am J Obstet Gynecol. 2008 Feb

Maternal Metabolic Control and Perinatal Outcome in Women With GDM Treated with Lispro or Aspart Insulin Comparison with regular insulin • They studied 96 women with GDM were randomly allocated to ASP (n = 31), LIS (n = 33), or HI (n = 32). • The three groups were comparable for age, parity, BMI, weight gain, and time of gestation. Blood glucose levels were measured five times daily. GRAZIANO DI CIANN Diabetes Care 30: e 11 2007

Results • At the end of pregnancy , no differences for insulin dose, weight gain, FPG , and A 1 C were registered • 1 -h PP level, was higher in HI patients (135 ± 23 mg/dl) than in LIS (118 ± 18 mg/dl) and ASP (121 ± 20 mg/dl) (ANOVA, P < 0. 05). • Birth weight was higher (P < 0. 04) in HI than in LIS and ASP patients. • Macrosomia resulted in 15. 6% of HI, 12. 1% of LIS, and 9. 6% of ASP (no statistical significance)

Conclusion It suggests that both short insulin analogs are associated with better postprandial maternal glucose control and anthropometric measures in newborns than HI. GRAZIANO DI CIANN Diabetes Care 30: e 11 2007

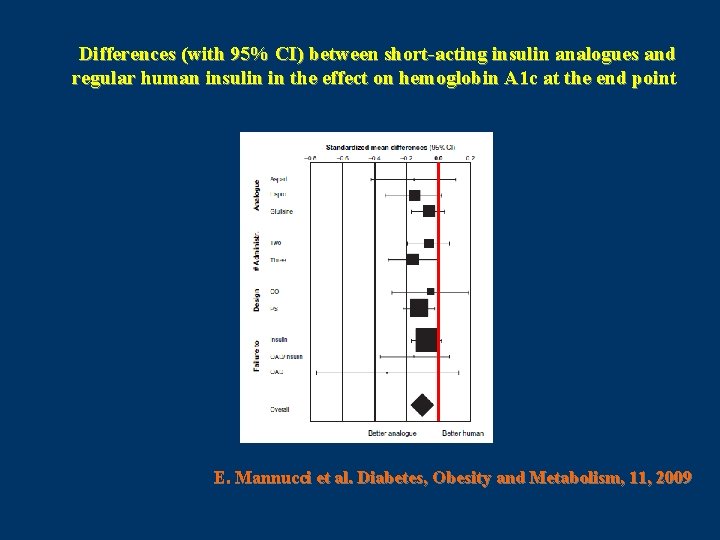
All randomized controlled trials (RCTs) with a duration >4 weeks comparing short-acting insulin analogues (lispro, aspart or glulisine) with HRI in type 2 diabetic patients were retrieved; data on Hb. A 1 c and postprandial glucose and incidence of severe hypoglycaemia were extracted and meta-analysed ( n = 13 RCT ) E. Mannucci et al. Diabetes, Obesity and Metabolism, 11, 2009

Differences (with 95% CI) between short-acting insulin analogues and regular human insulin in the effect on hemoglobin A 1 c at the end point E. Mannucci et al. Diabetes, Obesity and Metabolism, 11, 2009

In conclusion, the use of short-acting insulin analogues in type 2 diabetic patients provides a better control of postprandial glucose and Hb. A 1 c in comparison with human insulin. E. Mannucci et al. Diabetes, Obesity and Metabolism, 11, 2009

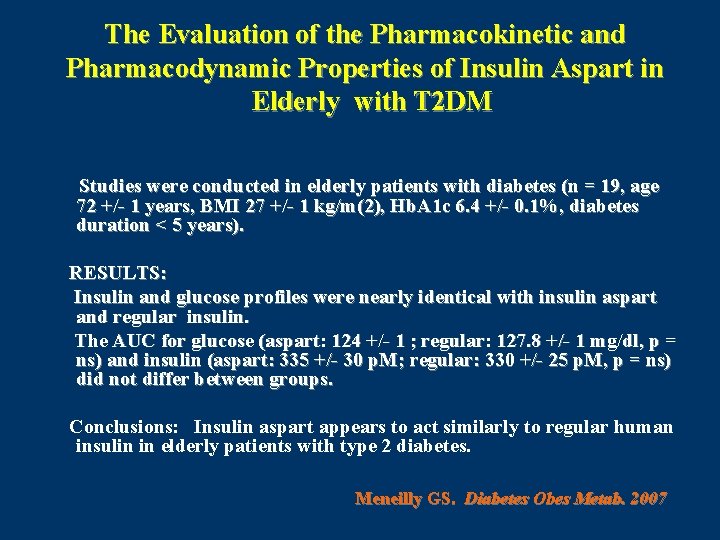
The Evaluation of the Pharmacokinetic and Pharmacodynamic Properties of Insulin Aspart in Elderly with T 2 DM Studies were conducted in elderly patients with diabetes (n = 19, age 72 +/- 1 years, BMI 27 +/- 1 kg/m(2), Hb. A 1 c 6. 4 +/- 0. 1%, diabetes duration < 5 years). RESULTS: Insulin and glucose profiles were nearly identical with insulin aspart and regular insulin. The AUC for glucose (aspart: 124 +/- 1 ; regular: 127. 8 +/- 1 mg/dl, p = ns) and insulin (aspart: 335 +/- 30 p. M; regular: 330 +/- 25 p. M, p = ns) did not differ between groups. Conclusions: Insulin aspart appears to act similarly to regular human insulin in elderly patients with type 2 diabetes. Meneilly GS. Diabetes Obes Metab. 2007

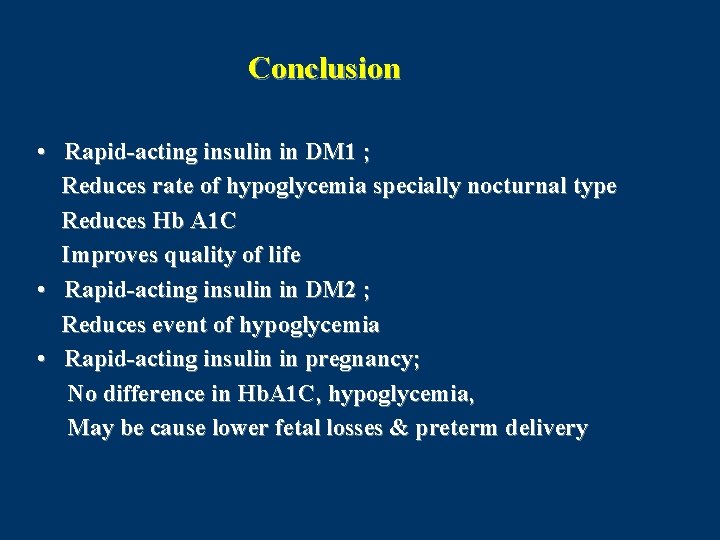
Conclusion • Rapid-acting insulin in DM 1 ; Reduces rate of hypoglycemia specially nocturnal type Reduces Hb A 1 C Improves quality of life • Rapid-acting insulin in DM 2 ; Reduces event of hypoglycemia • Rapid-acting insulin in pregnancy; No difference in Hb. A 1 C, hypoglycemia, May be cause lower fetal losses & preterm delivery

Conclusion Insulin treatment has always been as much an art as a science. The proper use of insulin analogues allows people with diabetes greater flexibility in the timing of meals, snacks, and exercise, which in turn enhances their ability to lead normal lives. When choosing between insulin analogues and RHI, not only should their differences in glycaemic control and treatment flexibility be considered, but also their oncogenic safety, cost of treatment, type of diet and patients’ preferences.

Thank you

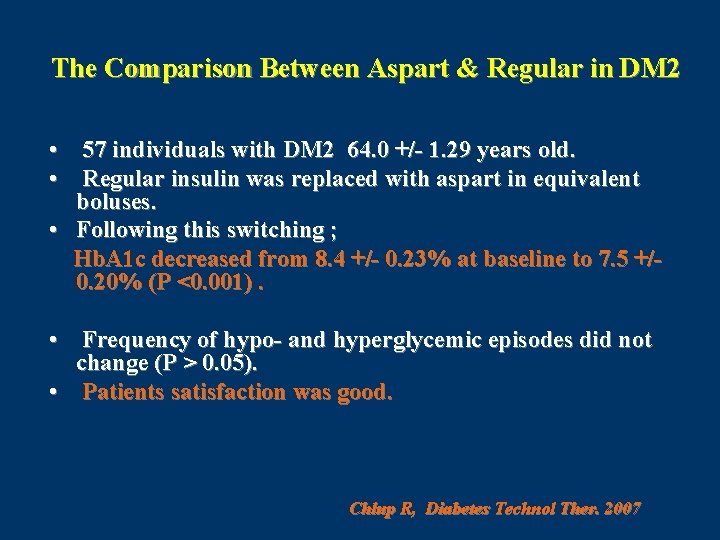
The Comparison Between Aspart & Regular in DM 2 • 57 individuals with DM 2 64. 0 +/- 1. 29 years old. • Regular insulin was replaced with aspart in equivalent boluses. • Following this switching ; Hb. A 1 c decreased from 8. 4 +/- 0. 23% at baseline to 7. 5 +/0. 20% (P <0. 001). • Frequency of hypo- and hyperglycemic episodes did not change (P > 0. 05). • Patients satisfaction was good. Chlup R, Diabetes Technol Ther. 2007

CONCLUSION Aspart appears to be more effective than human regular insulin for complementary insulin treatment in individuals with type 2 diabetes. Chlup R, Diabetes Technol Ther. 2007

• Insulin analogues have full biologic activity but less tendency for self-aggregation, resulting in more rapid absorption and onset of action and a shorter duration of action. • In general, treatment with monomeric insulin analogues (lispro, aspart, and glulisine) is associated with a lower risk of hypoglycemia, particularly in sleep, than treatment with regular insulin. • Finally, patients may inject these insulin analogues immediately before or after meals instead of 30 to 60 minutes before meals, as is classically recommended with regular insulin, providing greater convenience.

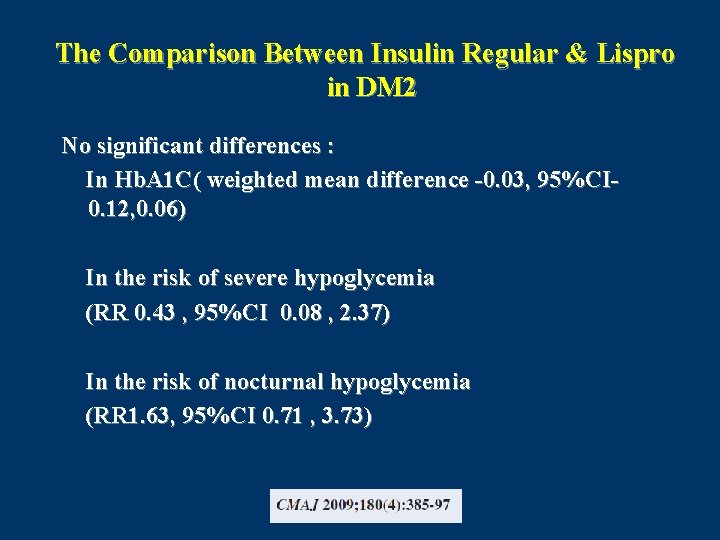
The Comparison Between Insulin Regular & Lispro in DM 2 No significant differences : In Hb. A 1 C( weighted mean difference -0. 03, 95%CI 0. 12, 0. 06) In the risk of severe hypoglycemia (RR 0. 43 , 95%CI 0. 08 , 2. 37) In the risk of nocturnal hypoglycemia (RR 1. 63, 95%CI 0. 71 , 3. 73)

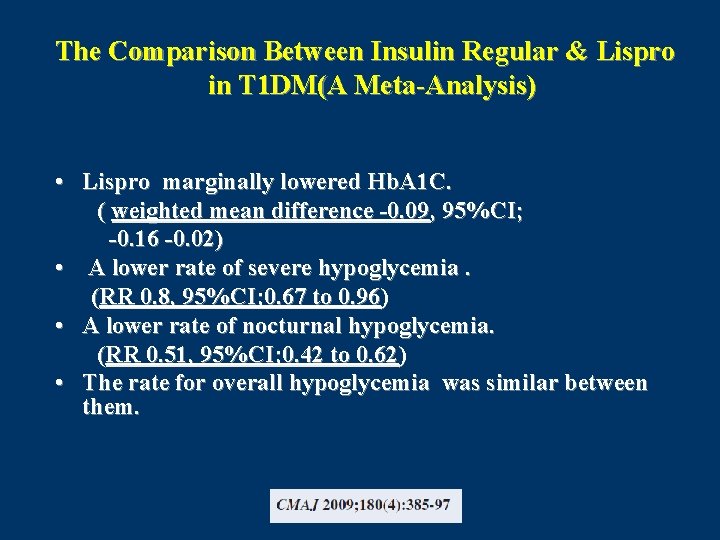
The Comparison Between Insulin Regular & Lispro in T 1 DM(A Meta-Analysis) • Lispro marginally lowered Hb. A 1 C. ( weighted mean difference -0. 09, 95%CI; -0. 16 -0. 02) • A lower rate of severe hypoglycemia. (RR 0. 8, 95%CI; 0. 67 to 0. 96) • A lower rate of nocturnal hypoglycemia. (RR 0. 51, 95%CI; 0. 42 to 0. 62) • The rate for overall hypoglycemia was similar between them.

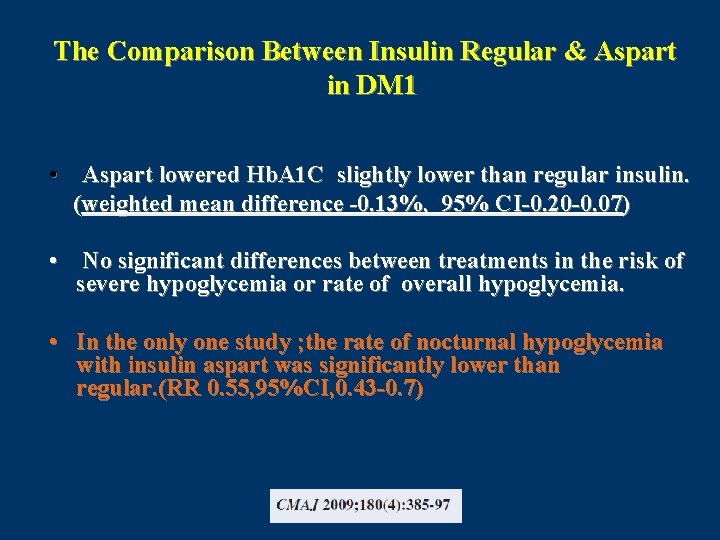
The Comparison Between Insulin Regular & Aspart in DM 1 • Aspart lowered Hb. A 1 C slightly lower than regular insulin. (weighted mean difference -0. 13%, 95% CI-0. 20 -0. 07) • No significant differences between treatments in the risk of severe hypoglycemia or rate of overall hypoglycemia. • In the only one study ; the rate of nocturnal hypoglycemia with insulin aspart was significantly lower than regular. (RR 0. 55, 95%CI, 0. 43 -0. 7)

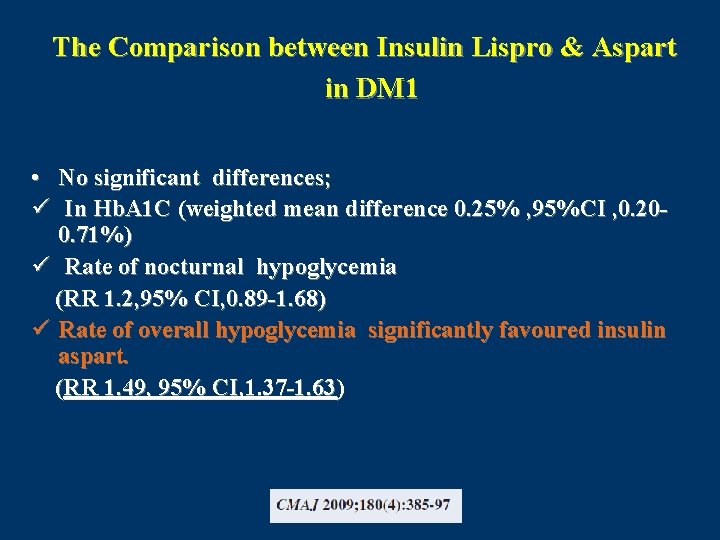
The Comparison between Insulin Lispro & Aspart in DM 1 • No significant differences; ü In Hb. A 1 C (weighted mean difference 0. 25% , 95%CI , 0. 200. 71%) ü Rate of nocturnal hypoglycemia (RR 1. 2, 95% CI, 0. 89 -1. 68) ü Rate of overall hypoglycemia significantly favoured insulin aspart. (RR 1. 49, 95% CI, 1. 37 -1. 63)

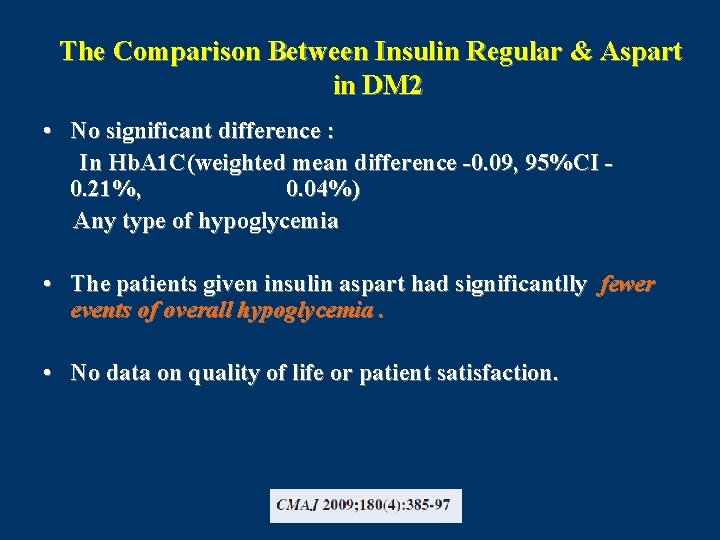
The Comparison Between Insulin Regular & Aspart in DM 2 • No significant difference : In Hb. A 1 C(weighted mean difference -0. 09, 95%CI 0. 21%, 0. 04%) Any type of hypoglycemia • The patients given insulin aspart had significantlly fewer events of overall hypoglycemia. • No data on quality of life or patient satisfaction.
 Name a line containing point a
Name a line containing point a Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Các số nguyên tố
Các số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Our god is an awesome god vine
Our god is an awesome god vine Our god is an awesome god
Our god is an awesome god Our god is an awesome god medley
Our god is an awesome god medley God is good god is great speed
God is good god is great speed O god you are my god earnestly i seek you
O god you are my god earnestly i seek you God-given virtues that direct us to our loving, triune god.
God-given virtues that direct us to our loving, triune god. Justice new testament
Justice new testament My god's bigger than your god
My god's bigger than your god Don't covet your neighbor's
Don't covet your neighbor's God's covenant
God's covenant Oh lord our lord how excellent is thy name in all the earth
Oh lord our lord how excellent is thy name in all the earth In the name of god amen
In the name of god amen In the name of god, most gracious, most merciful prayer
In the name of god, most gracious, most merciful prayer 馮定華神父
馮定華神父 Mercury god name
Mercury god name In the name of god the beneficent the merciful
In the name of god the beneficent the merciful In the name of god the beneficent the merciful
In the name of god the beneficent the merciful



















































































