Euromet 2006 Influence of impurities on the melting
















- Slides: 52

Euromet 2006 Influence of impurities on the melting temperature of Aluminum Pr. B. Legendre & Dr S. Fries EA 401

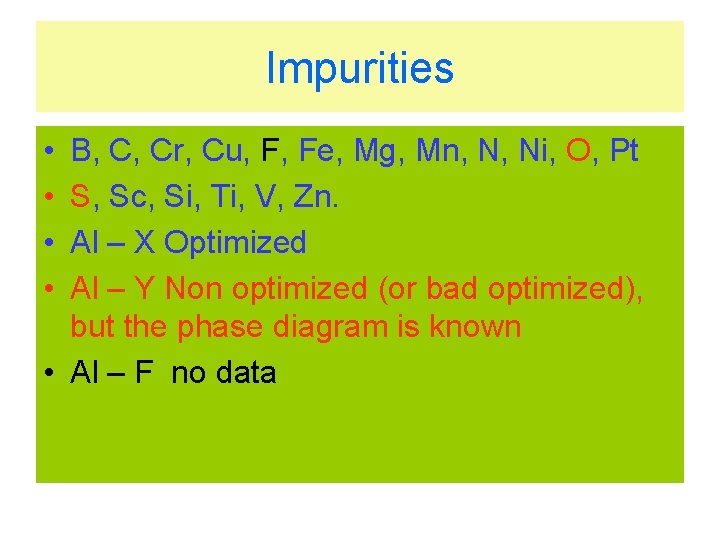
Impurities • • B, C, Cr, Cu, F, Fe, Mg, Mn, N, Ni, O, Pt S, Sc, Si, Ti, V, Zn. Al – X Optimized Al – Y Non optimized (or bad optimized), but the phase diagram is known • Al – F no data

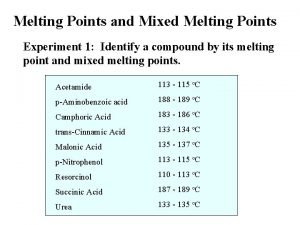
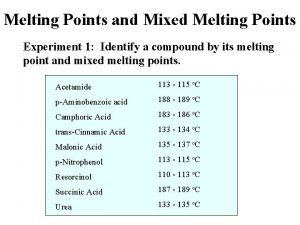
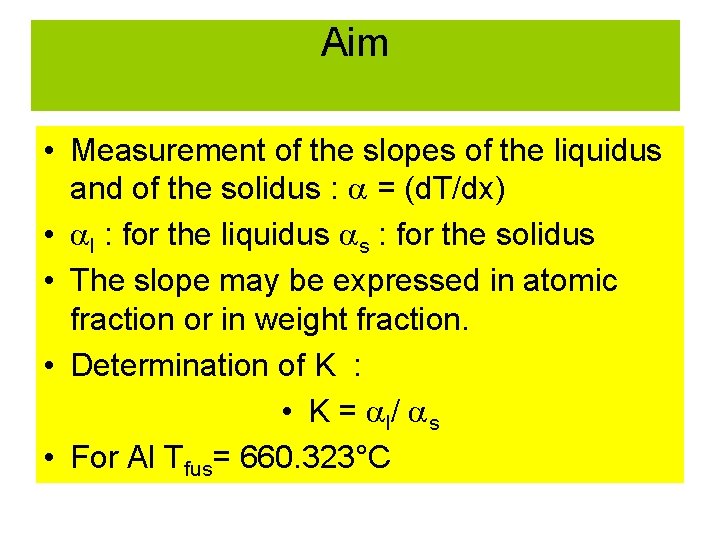
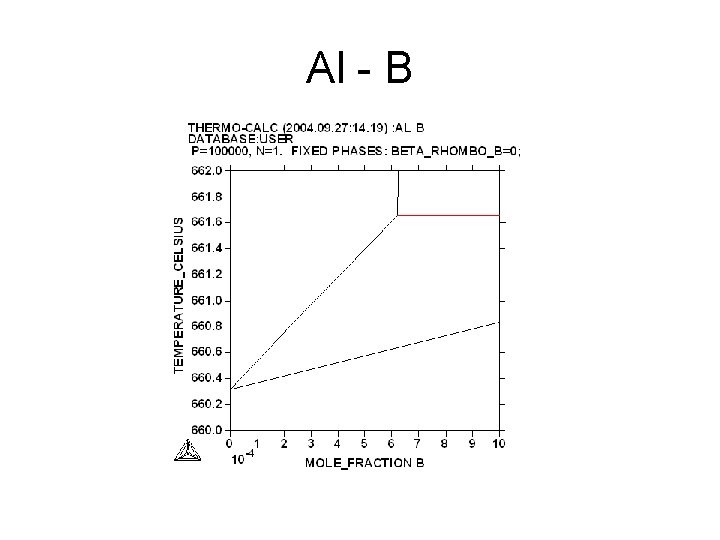
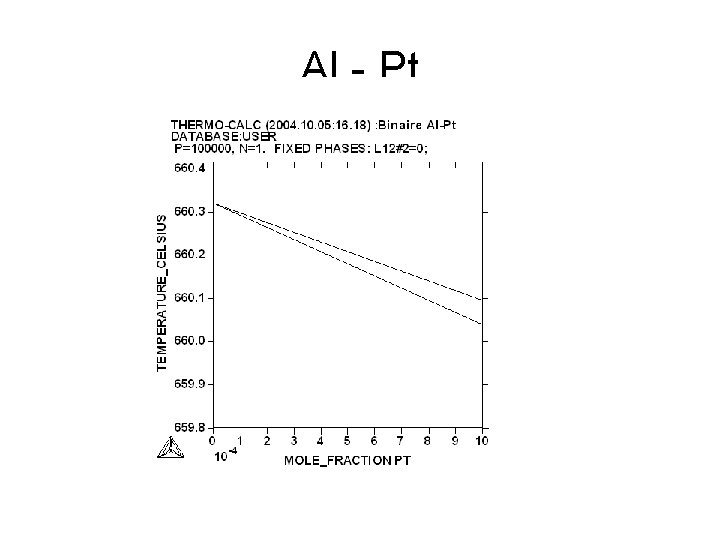
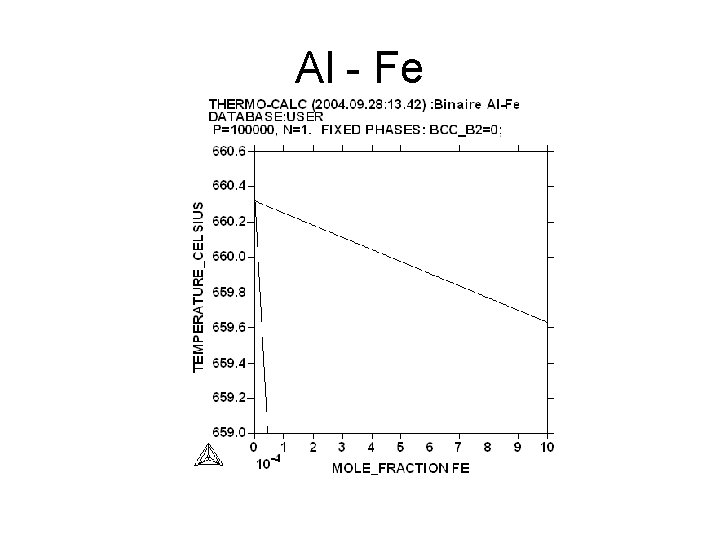
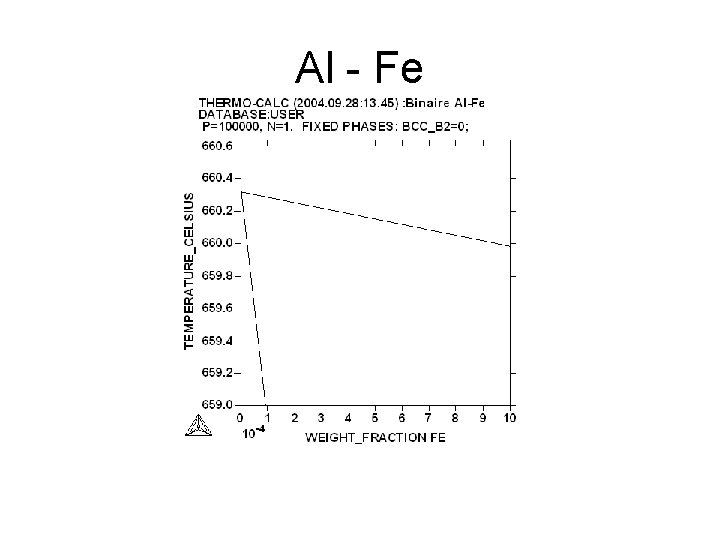
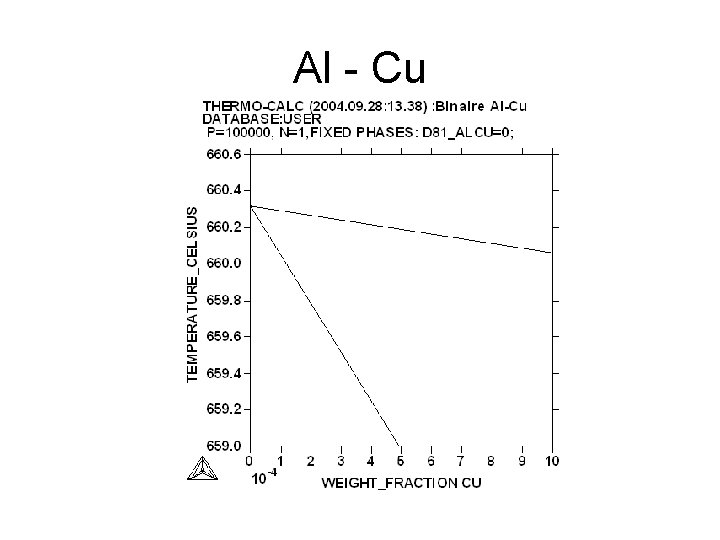
Aim • Measurement of the slopes of the liquidus and of the solidus : = (d. T/dx) • l : for the liquidus s : for the solidus • The slope may be expressed in atomic fraction or in weight fraction. • Determination of K : • K = l/ s • For Al Tfus= 660. 323°C

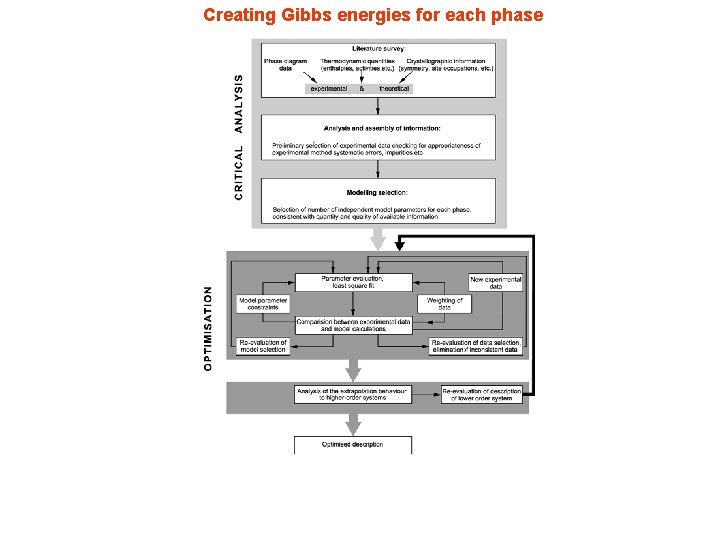
Phase diagram • For this subject we are interested only by a very small region in temperature and composition of binaries Al-X, but it is absolutely necessary to have a perfect knowledge of the full diagram. And to know the Gibbs function versus of T and x for each phase at a fixed pressure.

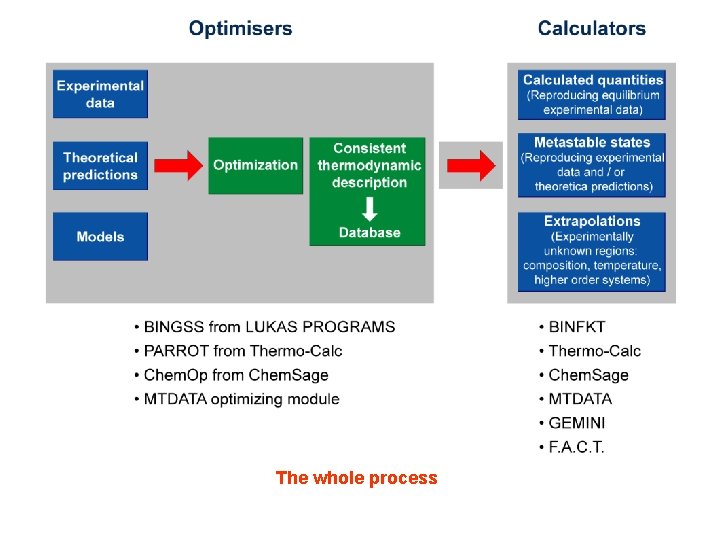
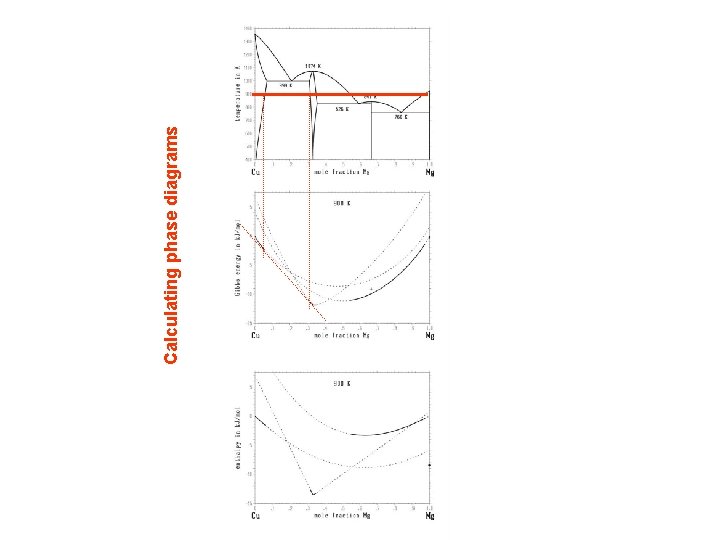
Phase diagram : Calphad Method • A phase diagram is the graphical expression of phase equilibria, for each phase in a binary system G = f(xi, T, P) • The limits of a two phase field are given by the common tangent of the G =f(xi)PT curves. • For a eutectic: • The three curves G=f(xi)p, T, have the same tangent. •

The whole process

Calculating phase diagrams

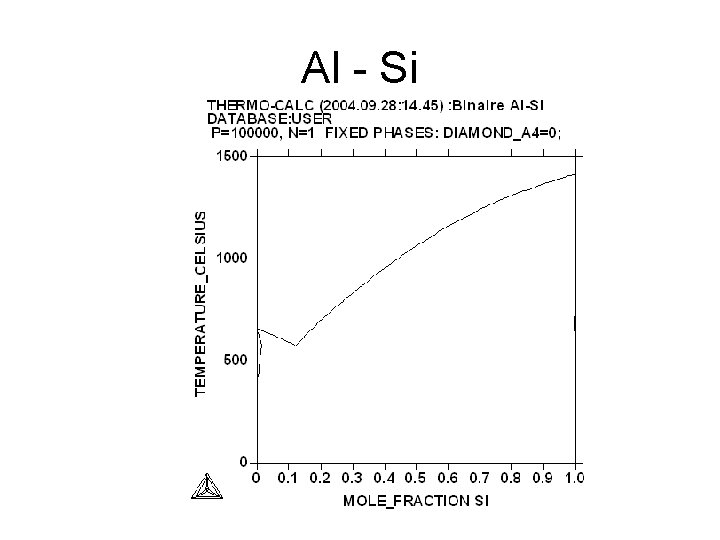
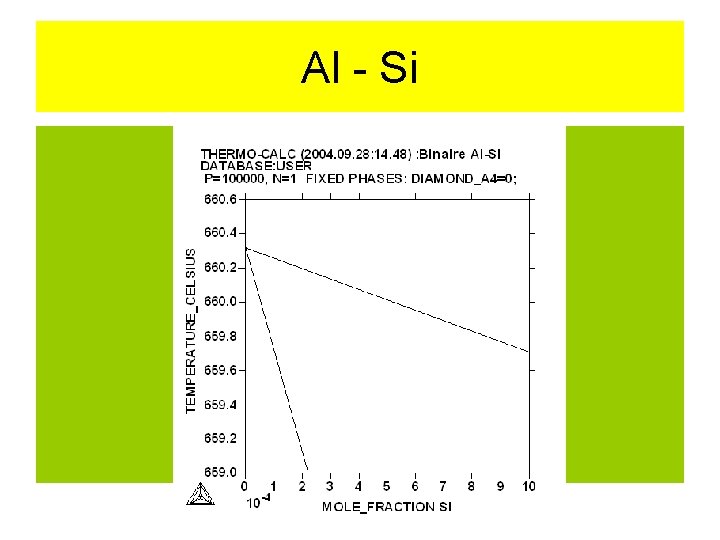
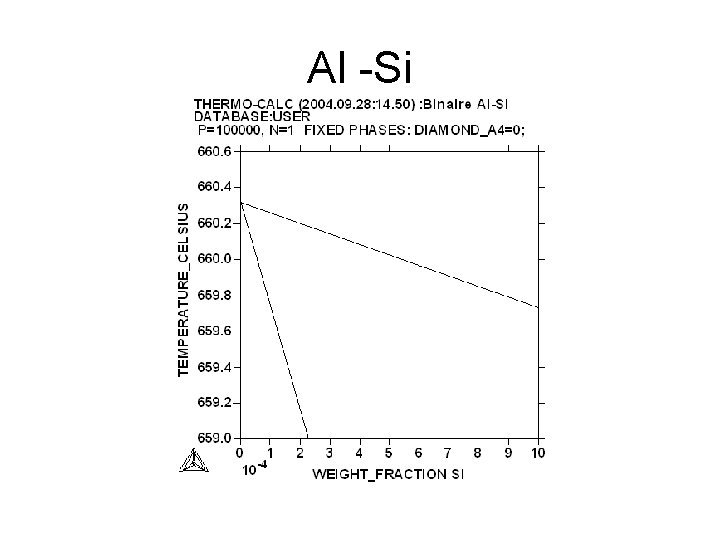
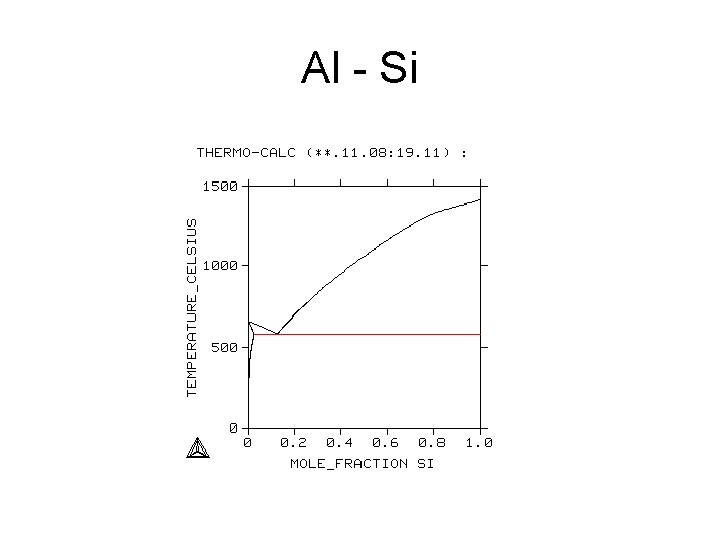
Al - Si

Al - Si

Al -Si

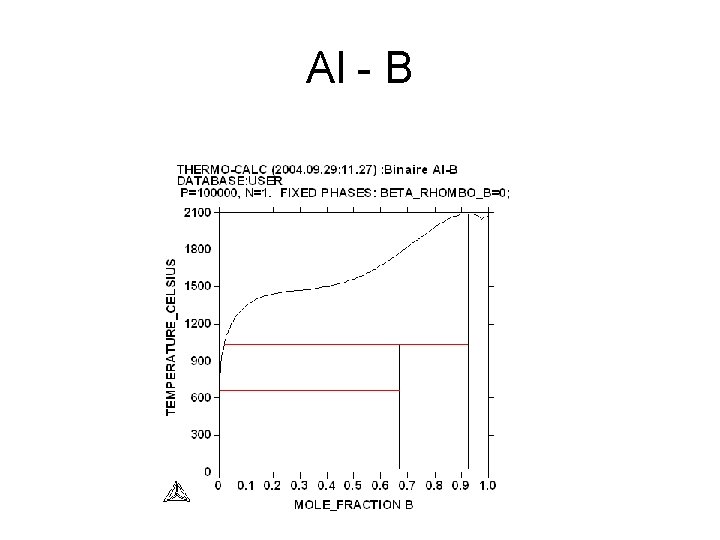
Al - B

Al - B

Al - B

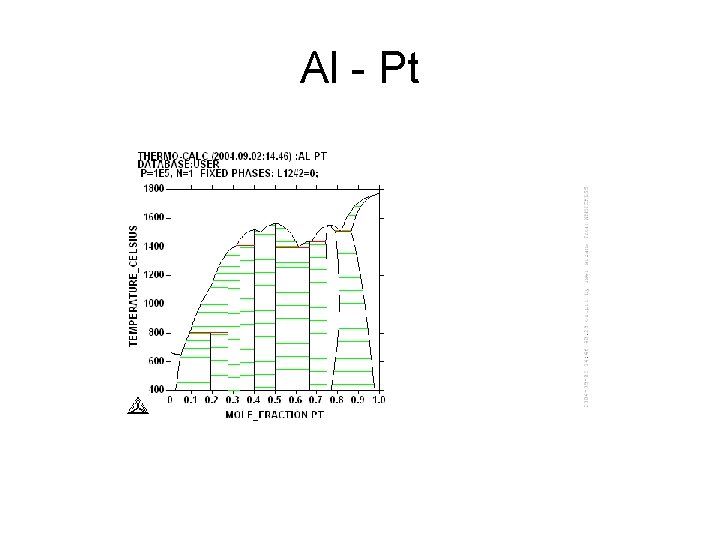
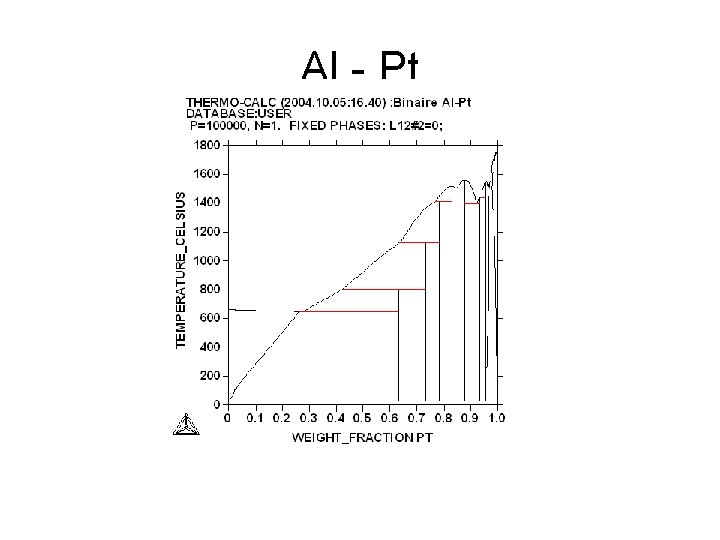
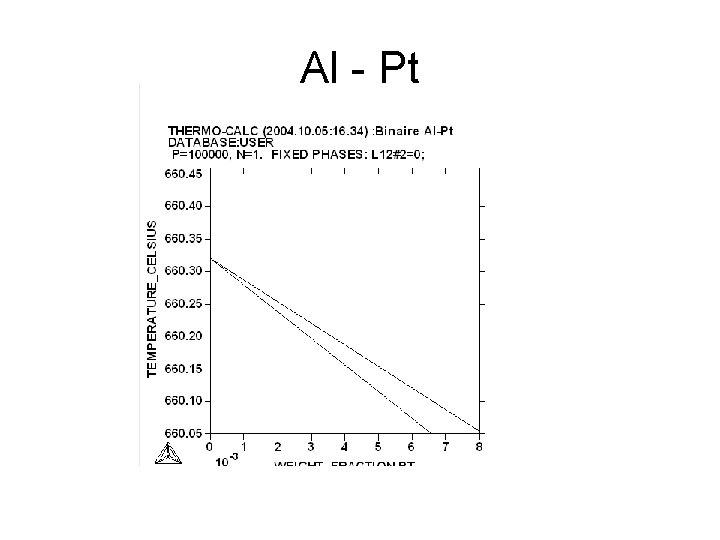
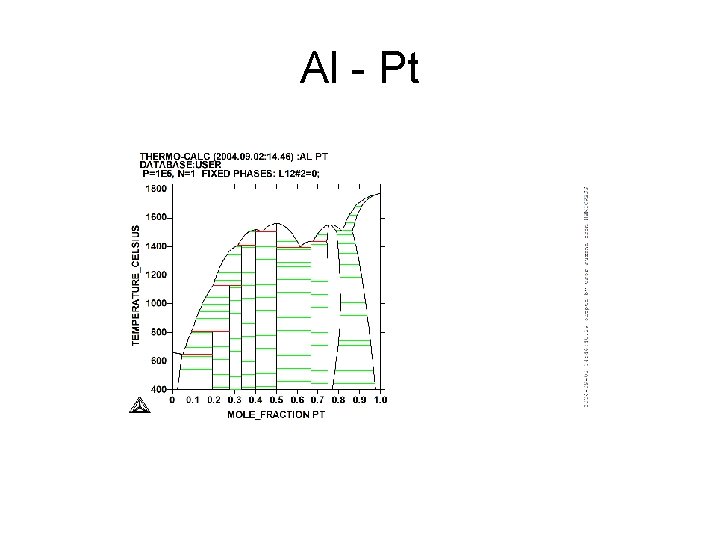
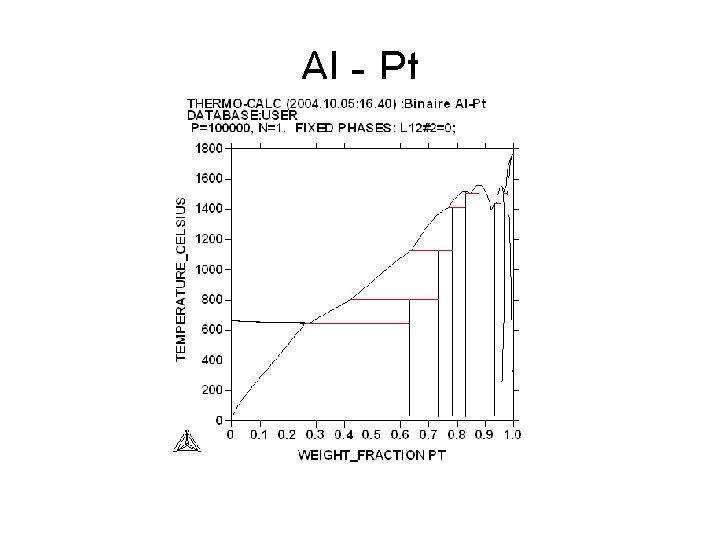
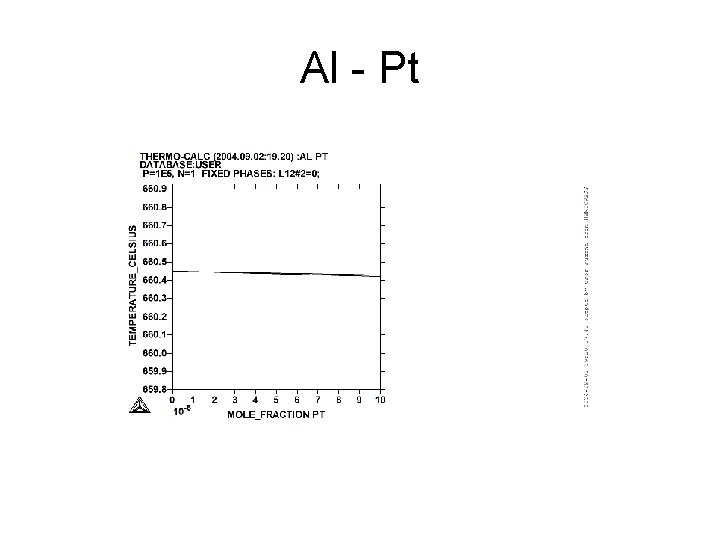
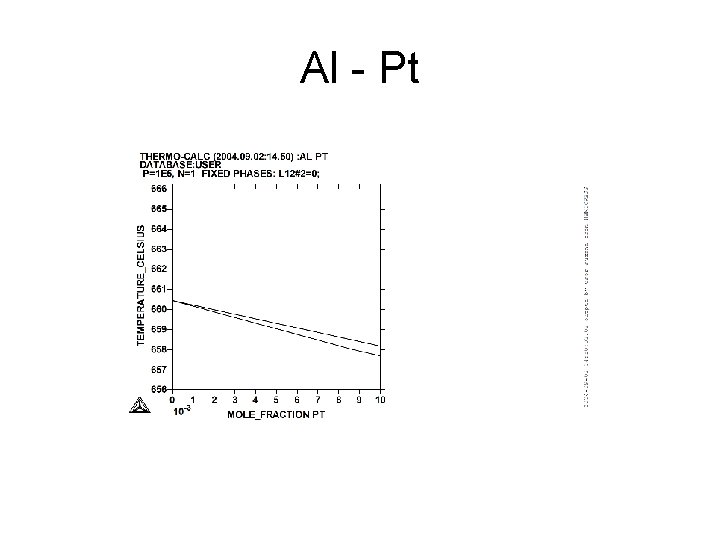
Al - Pt

Al - Pt

Al - Pt

Al - Pt

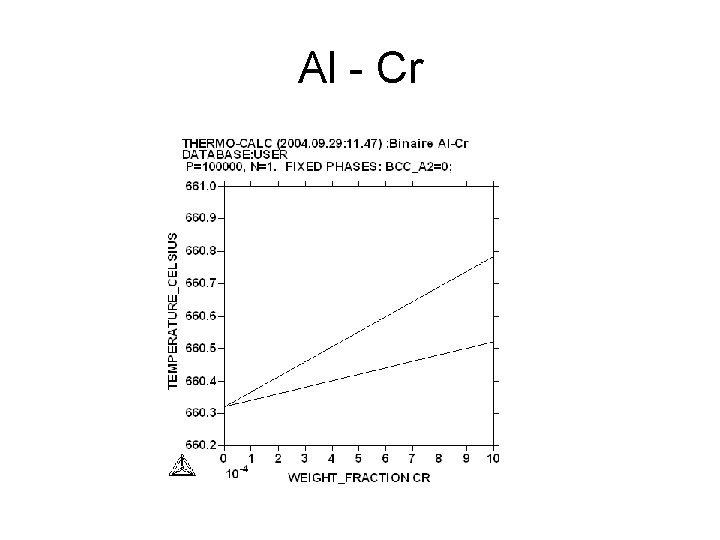
Al - Cr

Al - Cr

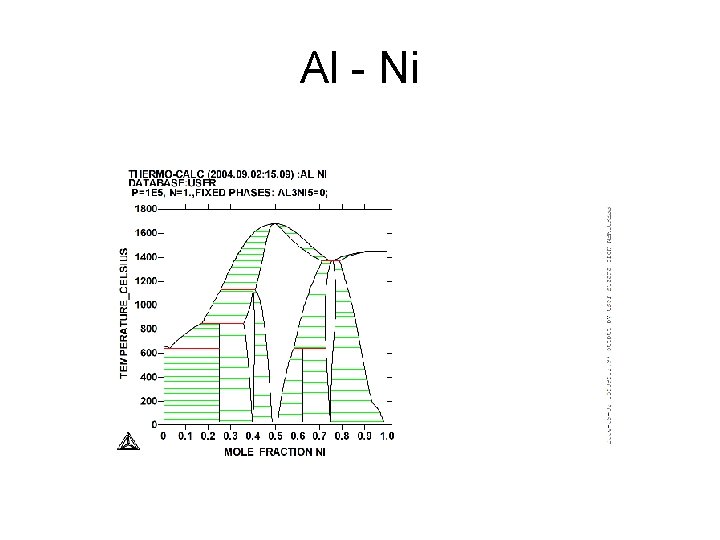
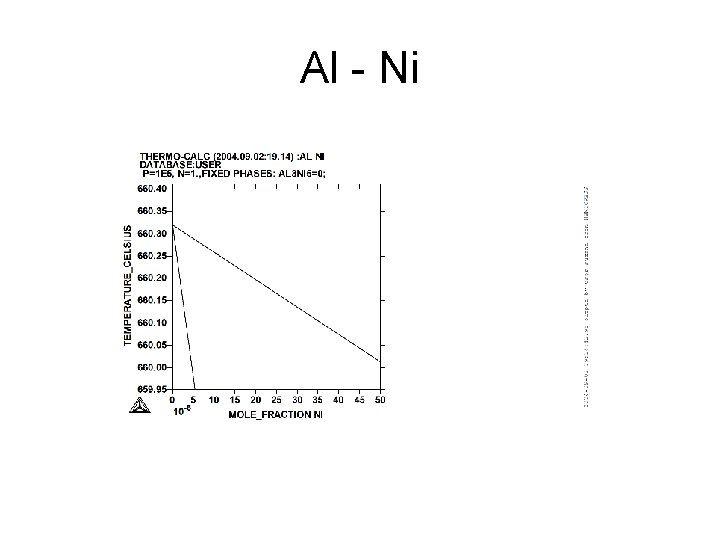
Al - Ni

Al - Ni

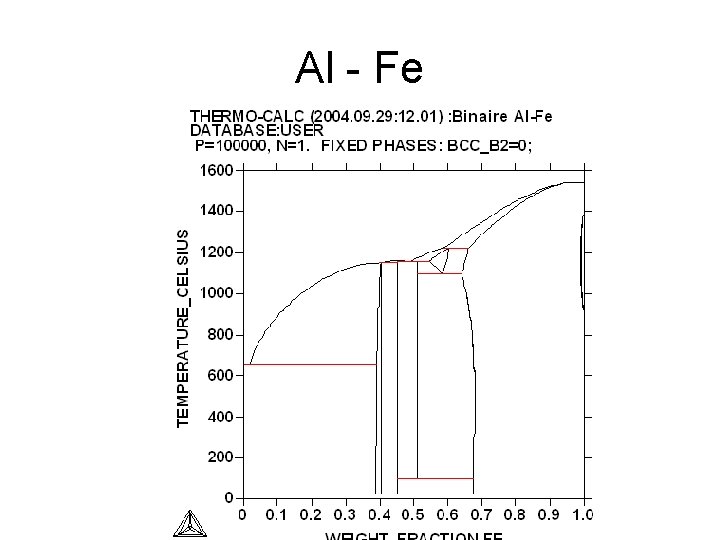
Al - Fe

Al - Fe

Al - Fe

Al - Cu

Al - Cu

Al - Cu

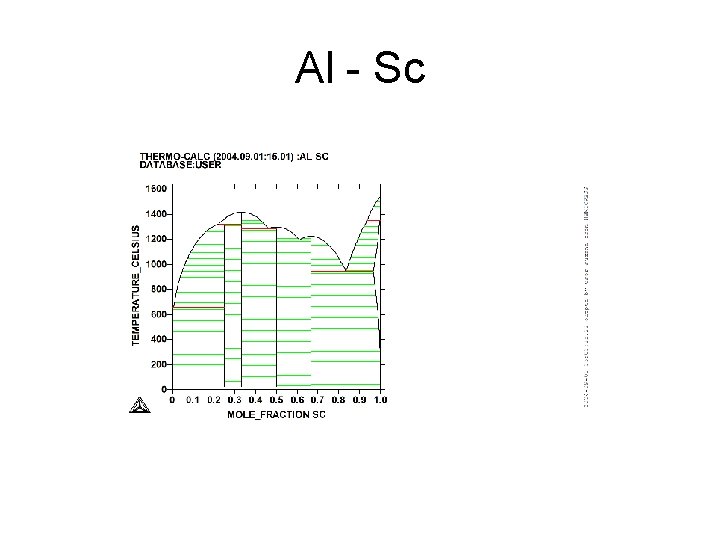
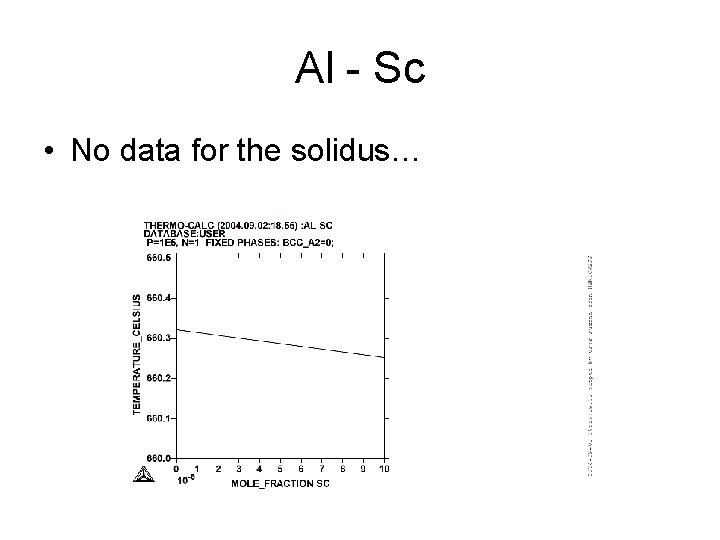
Al - Sc

Al - Sc • No data for the solidus…

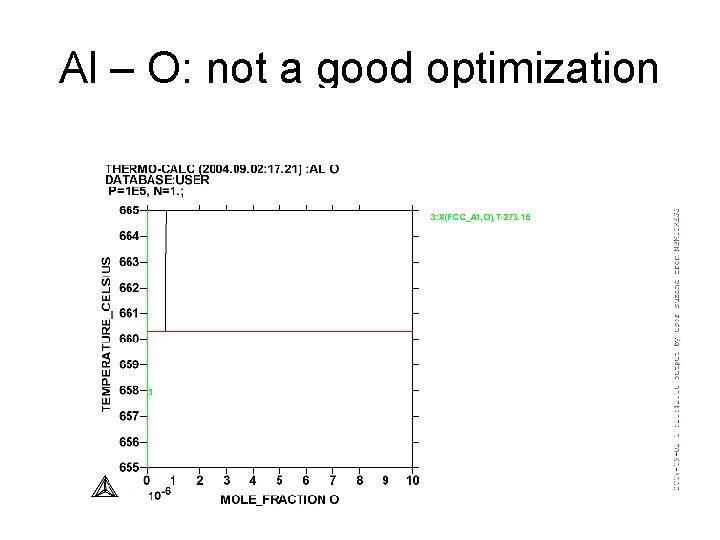
Al – O: not a good optimization


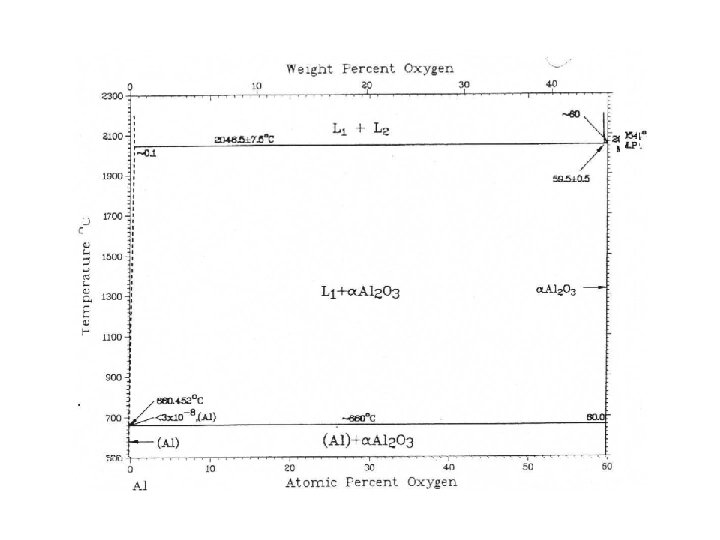
Al - O • Why it is not possible to have a good optimization of the Al-O binary? • The oxygen forms with Al an oxide which is a thin film fixed on the surface of Al and then it is a kind of protection (aluminum pans are used on the gas in a kitchen…) after the fixation of a first layer the oxygen cannot go deeper in Al. There is no homogeneity and no equilibrium.

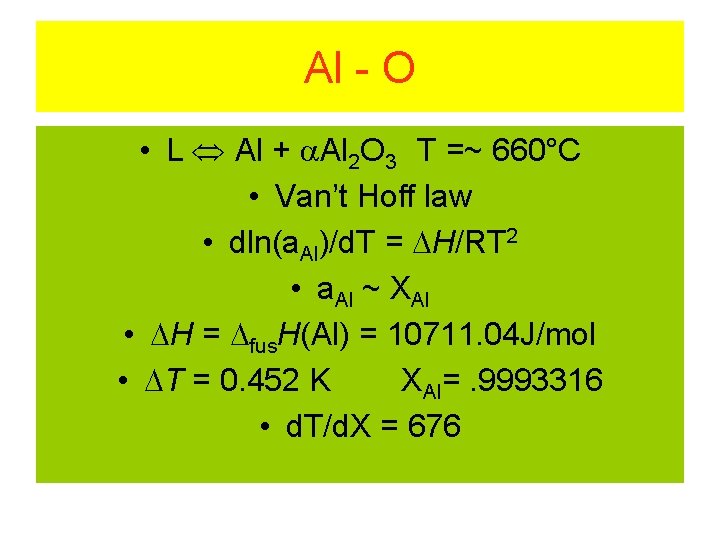
Al - O • L Al + Al 2 O 3 T =~ 660°C • Van’t Hoff law • dln(a. Al)/d. T = H/RT 2 • a. Al ~ XAl • H = fus. H(Al) = 10711. 04 J/mol • T = 0. 452 K XAl=. 9993316 • d. T/d. X = 676

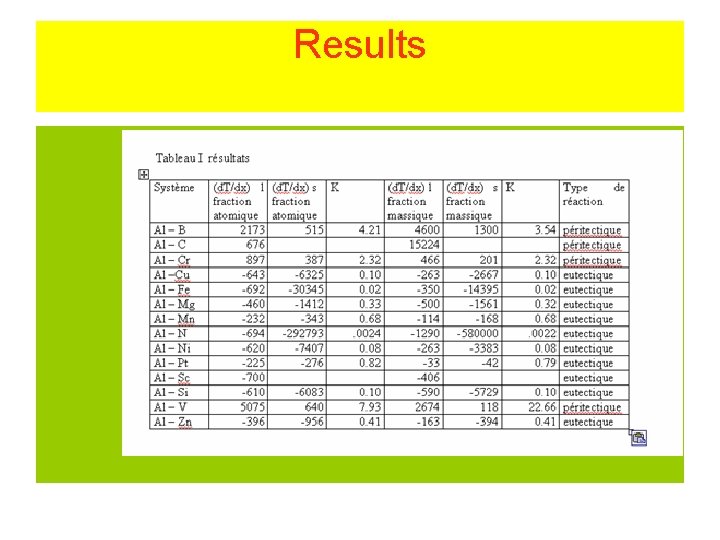
Results

Comparison of results

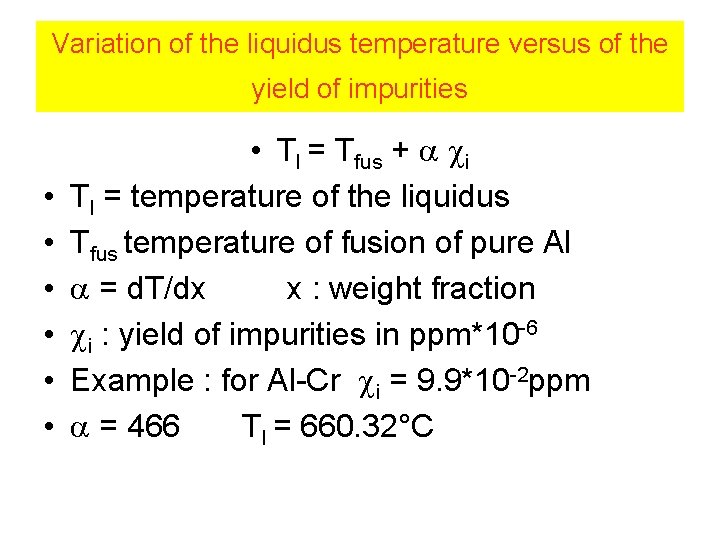
Variation of the liquidus temperature versus of the yield of impurities • • Tl = Tfus + i Tl = temperature of the liquidus Tfus temperature of fusion of pure Al = d. T/dx x : weight fraction i : yield of impurities in ppm*10 -6 Example : for Al-Cr i = 9. 9*10 -2 ppm = 466 Tl = 660. 32°C

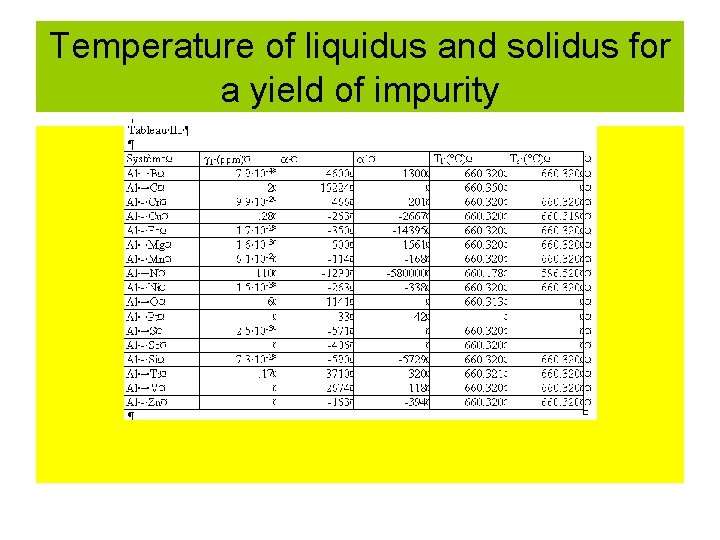
Temperature of liquidus and solidus for a yield of impurity

Conclusions • It has been possible to determine the influence of impurities in Al for nearly all the selected elements. • For O and S just an estimation has been possible. • The most important modifications occurs with O and N • It is possible to perform a calculation with two impurities.

Acknowledgement • This work has been financially supported by LNE and BNM • We are very grateful to these organisms for their contribution. •





Al - Pt

Al - Pt

Al - Pt

Al - Ni


Al - Pt

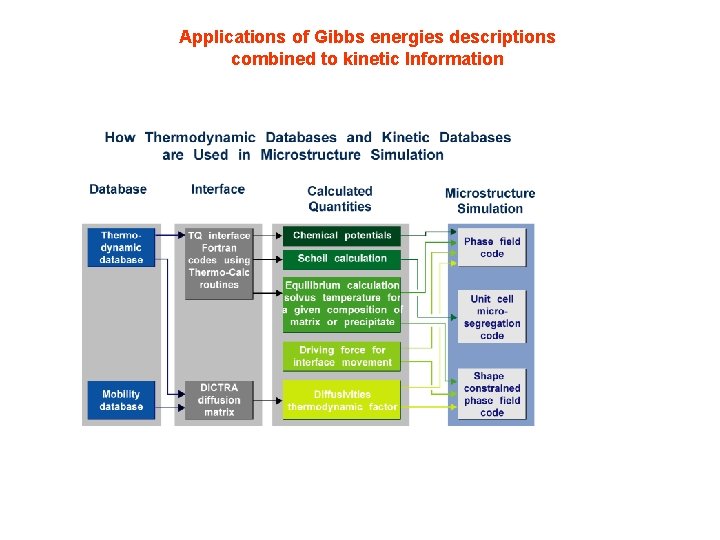
Applications of Gibbs energies descriptions combined to kinetic Information

Creating Gibbs energies for each phase

Al - Si
 Euromet share price
Euromet share price Incongruently melting compound
Incongruently melting compound Which grand sauce is made from milk and white roux
Which grand sauce is made from milk and white roux Desvenlafaxine medscape
Desvenlafaxine medscape Impurities in ceramics
Impurities in ceramics Trivalent and pentavalent impurities
Trivalent and pentavalent impurities The diffused impurities with
The diffused impurities with Common solvent impurities
Common solvent impurities Examples of donor impurities
Examples of donor impurities Tư thế ngồi viết
Tư thế ngồi viết đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Tư thế ngồi viết
Tư thế ngồi viết ưu thế lai là gì
ưu thế lai là gì Chó sói
Chó sói Thẻ vin
Thẻ vin Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Ví dụ về giọng cùng tên
Ví dụ về giọng cùng tên Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau 101012 bằng
101012 bằng Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Lời thề hippocrates
Lời thề hippocrates Chụp tư thế worms-breton
Chụp tư thế worms-breton đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Cong thức tính động năng
Cong thức tính động năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ So nguyen to
So nguyen to Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người Melting pot and salad bowl theory
Melting pot and salad bowl theory Batch melting
Batch melting