Determination of melting points and mixed melting points







- Slides: 20

Determination of melting points and mixed melting points

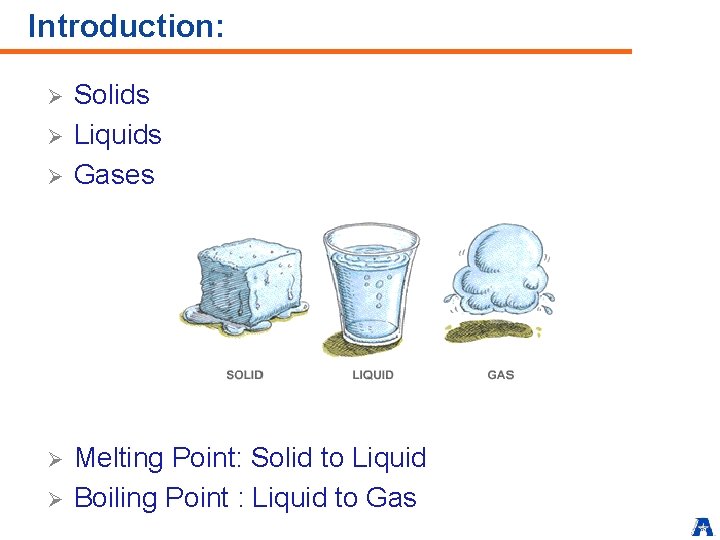
Introduction: Ø Ø Ø Solids Liquids Gases Melting Point: Solid to Liquid Boiling Point : Liquid to Gas

Introduction Melting point of a solid compound Ø Temperature at which a phase transition from solid to liquid occurs Melting point of a pure crystalline compound Ø Temperature at which thermal energy is sufficient to break down the crystal lattice of the solid Ø Sharply defined Ø Highly reproducible

mp Measurements Ø Very precise mps have been measured by determining the temperature at which the pure liquid and pure solid are in equilibrium – e. g. H 2 O Ø Routine work – Observe the temperature range – Temperature range for the first appearance of liquid to the final disappearance of solid – Heat a small sample of solid contained in a narrow glass capillary tube

Effect of impurities in a sample Ø Melting point of the sample is depressed relative to that of the pure substance Ø mp range is often off by a substantial amount Ø This method used to test whether two separate samples having similar appearance and melting points are really samples of the same compound, or if the similarity is merely coincidental.

Mixed melting points Ø Mix together small amounts of the two samples and determine the melting point of the mixture. Ø If the two samples are really the same compound, then mixing them will not change the melting point. Ø If the two samples are different compounds, then mixing them will produce an impure substance, – characteristic depression and – broadening of the melting point that is expected for an impure sample. Ø This mixed melting point technique will be used to identify an “unknown” compound.

Measuring melting points Ø Mel-Temp apparatus will be used to determine melting points.

Sample preparation Ø Fill the solid compound into the capillary tube through open end. Ø Only 2 -4 mm of sample is required. Ø Do not over fill ‒ poor heat transfer can cause inaccuracies Place the tube in a capillary well with aluminum block below 70 ºC Ready to measure the mp Ø Ø

Measuring the mp Ø If you know the expected MP of your sample set the voltage to provide appropriate heating rate Ø Initial heating rate may be fairly fast Ø Heating ~10 -20 o. C per min. Ø When thermometer reading is about 15 -20 o. C below the mp, turn the voltage down Ø When melting, the heating rate must be ~ 2 -3 o. C per min. for accurate measurements.

Part A- mp of pure compounds(work in pairs) Ø Choose two compounds with similar mp’s Ø Measure the mp Ø Record the temperature range where melting is observed Ø Compare with the literature values with table Ø Values must agree within ± 2 o. C

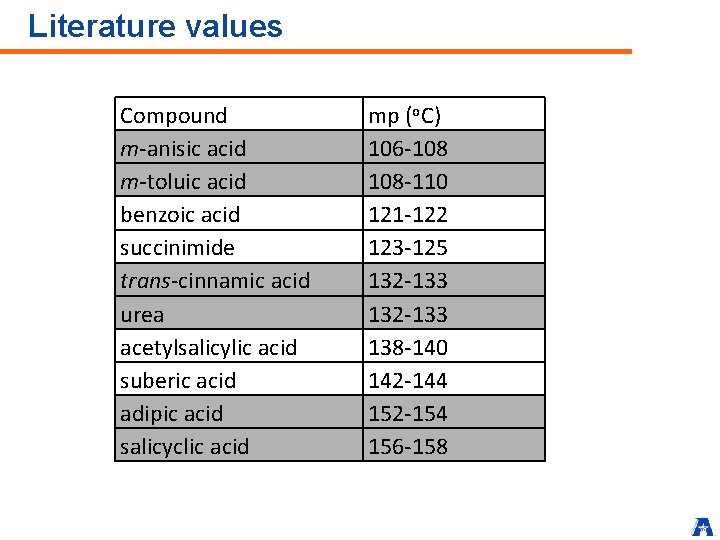
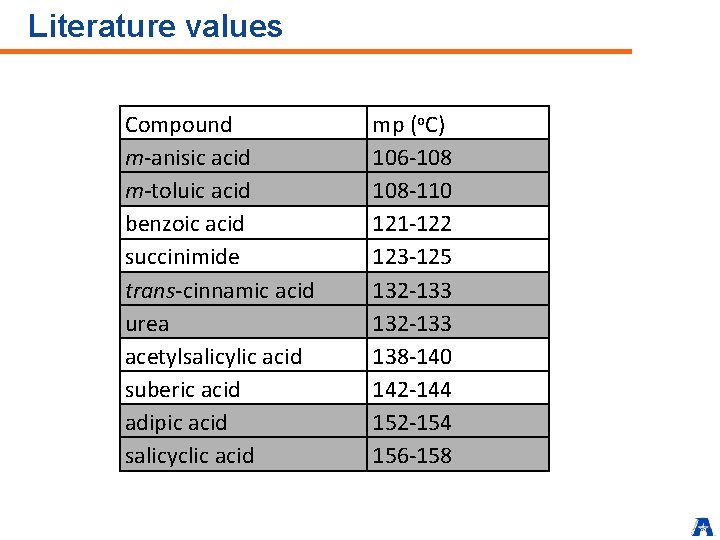
Literature values Compound m-anisic acid m-toluic acid benzoic acid succinimide trans-cinnamic acid urea acetylsalicylic acid suberic acid adipic acid salicyclic acid mp (o. C) 106 -108 108 -110 121 -122 123 -125 132 -133 138 -140 142 -144 152 -154 156 -158

B. Mixed mp of known compounds (pairs) Ø Use the same two reference samples you chose for part A. Ø Combine equal quantities of the two samples on a watch glass Ø Grind them together thoroughly, but carefully Ø Prepare the capillary tube for analysis Ø Measure and record the mp range of the mixture

C. Identification of an unknown sample (individually) Ø Target : determination of an unknown (Which reference standard corresponds to your unknown sample? ) Ø Obtain an unknown sample from your instructor Ø Prepare two capillary tubes of the pure sample

Rapid determination of approximate mp of unknown (i) Ø Place one sample in the Mel-temp apparatus Ø Set the voltage to give a fairly rapid heating rate (50 -60 volts) Ø Record the mp range

Accurate determination of approximate mp of unknown (ii) Ø Place the second capillary in the mp apparatus Ø Start to measure at a temperature 15 -20 o. C below the approximate value found in “A” Ø Set the voltage to give 2 -3 o. C per min heating rate when melting is actually occurring

Listing possible compounds (iii) Ø Assume that your unknown is probably one of compounds in Table Ø Compounds have literature mp within ± 5 o. C of your observed value Ø List those two compounds along with their literature mp values

Literature values Compound m-anisic acid m-toluic acid benzoic acid succinimide trans-cinnamic acid urea acetylsalicylic acid suberic acid adipic acid salicyclic acid mp (o. C) 106 -108 108 -110 121 -122 123 -125 132 -133 138 -140 142 -144 152 -154 156 -158

Mixed melting point determination and conclusions Ø Obtain samples of each reference standard listed with similar mp’s to your unknown Ø Run mixed mps of each of these standards with your unknown sample Ø On the basis of your observations identify your unknown compound

Caution Ø Some of the chemicals used in this experiment are irritants and should never be handled with bare hands. Ø Aluminum block of Mel-Temp becomes hot when in use. Do not touch. Ø Turn the voltage down when you finish each sample. Ø Thermometers are fragile and may crack if cooled too rapidly. Do not place hot thermometer directly on lab bench.

Waste Disposal Ø Ø Capillaries: Place in broken glassware containers Solids: Any extra solid should be disposed into the solid waste container