Electronic structure Atomic structure Atoms neutral Protons have






















- Slides: 62

Electronic structure

Atomic structure

Atoms: neutral • Protons have a positive charge and electrons have a negative charge. • Atoms are neutral so they have the same number of electrons as protons. • The protons are in the nucleus and do not change or vary except in some nuclear reactions. • The electrons are in discrete pathways or shells around the nucleus.

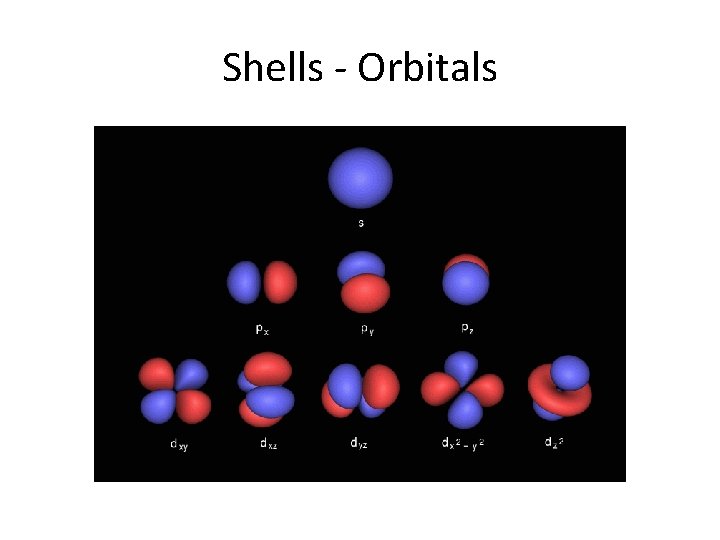
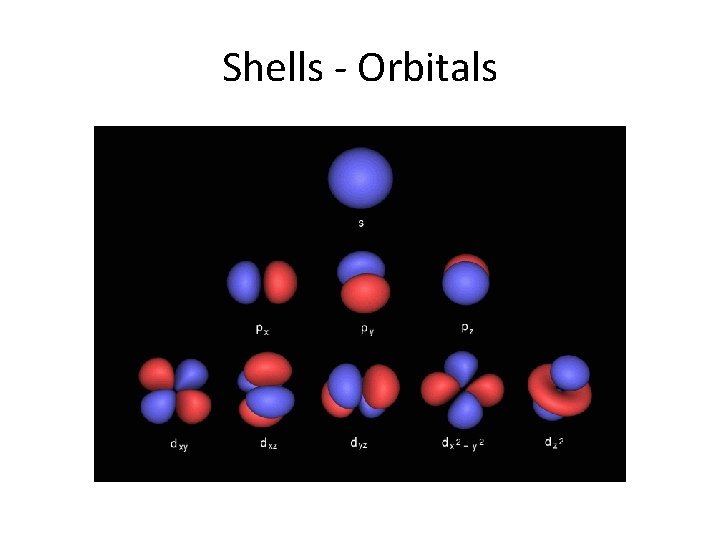
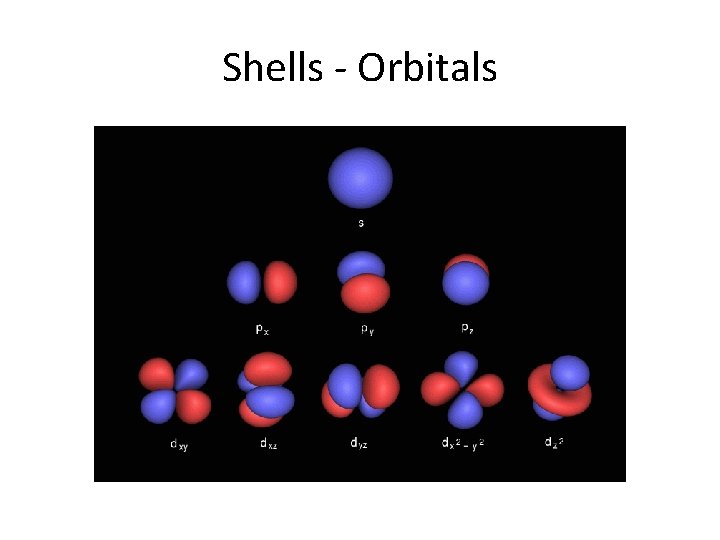
Shells - Orbitals

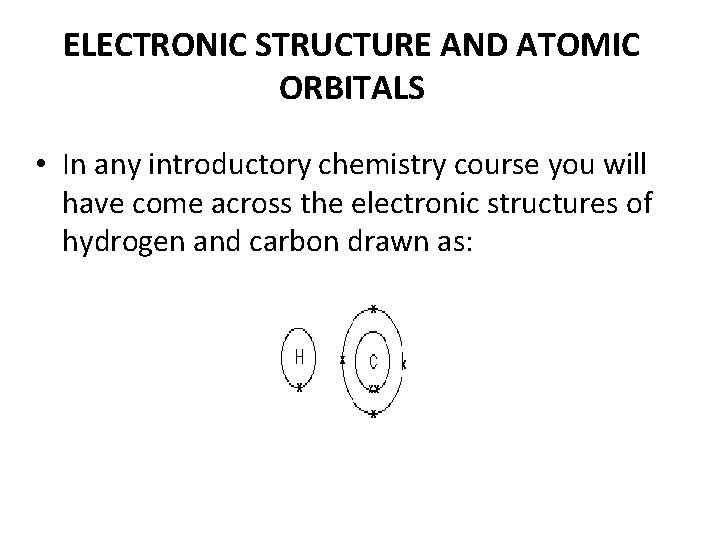
ELECTRONIC STRUCTURE AND ATOMIC ORBITALS • In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as:

Hydrogen's electron - the 1 s orbital Suppose you had a single hydrogen atom and at a particular instant plotted the position of the one electron. Soon afterwards, you do the same thing, and find that it is in a new position. You have no idea how it got from the first place to the second. You keep on doing this over and over again, and gradually build up a sort of 3 D map of the places that the electron is likely to be found.

Names of orbitals • Each orbital has a name. • The orbital occupied by the hydrogen electron is called a 1 s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. • The "s" tells you about the shape of the orbital. s orbitals are spherically symmetric around the nucleus - in each case, like a hollow ball with the nucleus at its centre.

2 s orbitals

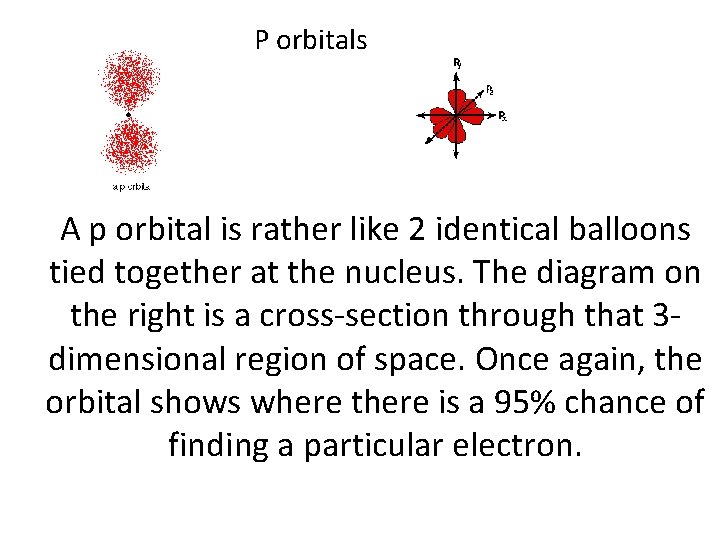
P orbitals A p orbital is rather like 2 identical balloons tied together at the nucleus. The diagram on the right is a cross-section through that 3 dimensional region of space. Once again, the orbital shows where there is a 95% chance of finding a particular electron.

P orbitals • At any one energy level it is possible to have three absolutely equivalent p orbitals pointing mutually at right angles to each other. These arbitrarily given the symbols px, py and pz. This is simply for convenience - what you might think of as the x, y or z direction changes constantly as the atom tumbles in space.

The electronic structure of hydrogen • Hydrogen has only one electron and that will go into the orbital with the lowest energy - the 1 s orbital. • Hydrogen has an electronic structure of 1 s 1. We have already described this orbital earlier. • The electron of a hydrogen atom travels around the proton nucleus in a shell of a spherical shape

Helium • Helium, element number two, has two electrons in the same spherical shape around the nucleus. • The first shell only has one subshell, and that subshell has only one orbital, for electrons. • Each orbital has a place for two electrons. The spherical shape of the lone orbital in the first energy level has given it the name s orbital.

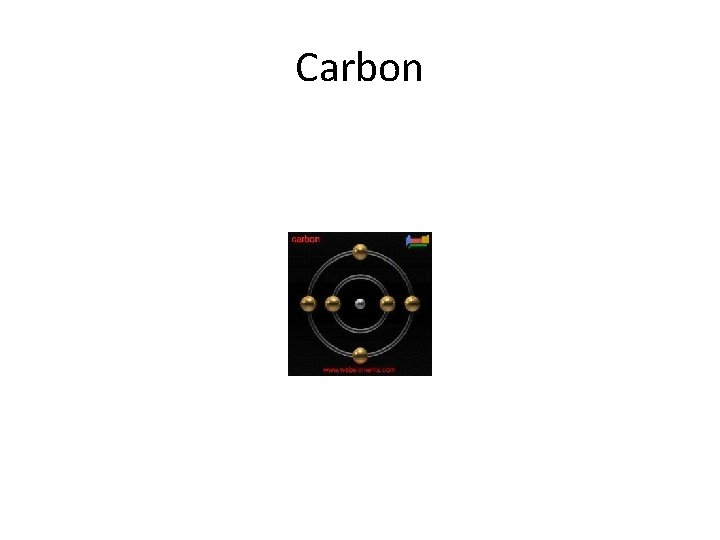
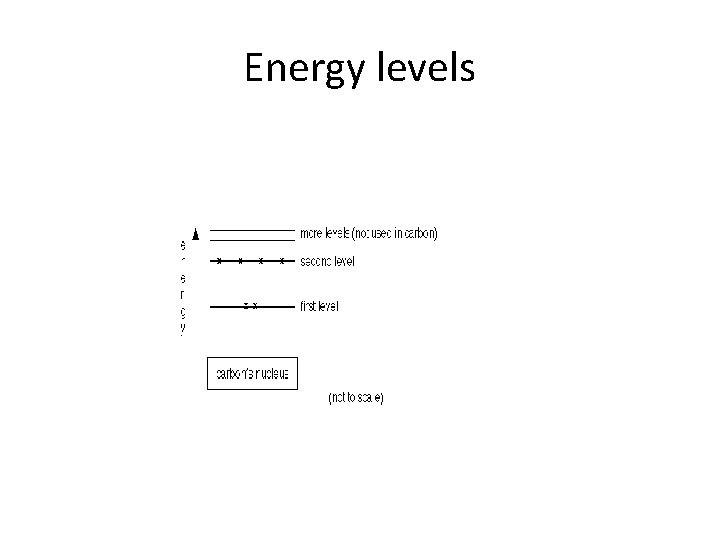
The electronic structure of carbon • Carbon has six electrons. Two of them will be found in the 1 s orbital close to the nucleus. The next two will go into the 2 s orbital. • The remaining ones will be in two separate 2 p orbitals. This is because the p orbitals all have the same energy and the electrons prefer to be on their own if that's the case. • The electronic structure of carbon is normally written 1 s 22 p 2

Carbon

Carbon • Name: Carbon • • • Atomic Number: 6 Chemical Symbol: C Electronic Configuration: 1 s 22 p 2 Atomic Mass: 12. 0 Atomic Ionization Energies: – 2 p : 1086 k. J mol-1 – 2 s : 1601 k. J mol-1 – 1 s : 27788 k. J mol-1

Orbitals, Shells • Physicists and chemists use a standard notation to describe the electron configurations of atoms and molecules. For atoms, we use atomic orbital labels (eg, 1 s, 3 d, 4 f) with the number of electrons assigned to each orbital placed as a superscript. For example: • hydrogen has one electron in the s-orbital of the first shell, so its configuration is written 1 s 1. • Lithium has two electrons in the 1 s-subshell and one in the (higher-energy) 2 s-subshell, so its configuration is written 1 s 2 2 s 1 (pronounced "one-ess-two, two-ess-one"). • Phosphorus (atomic number 15), is as follows: 1 s 2 2 p 6 3 s 2 3 p 3.

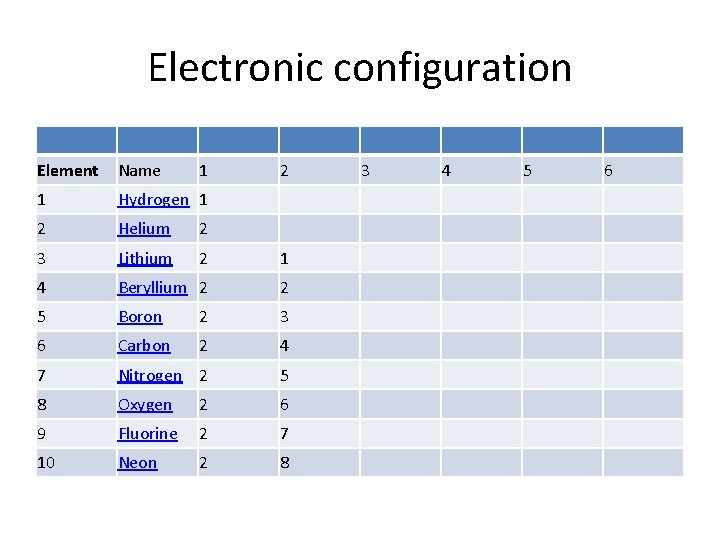
Electronic configuration Element Name 1 2 1 Hydrogen 1 2 Helium 2 3 Lithium 2 1 4 Beryllium 2 2 5 Boron 2 3 6 Carbon 2 4 7 Nitrogen 2 5 8 Oxygen 2 6 9 Fluorine 2 7 10 Neon 2 8 3 4 5 6

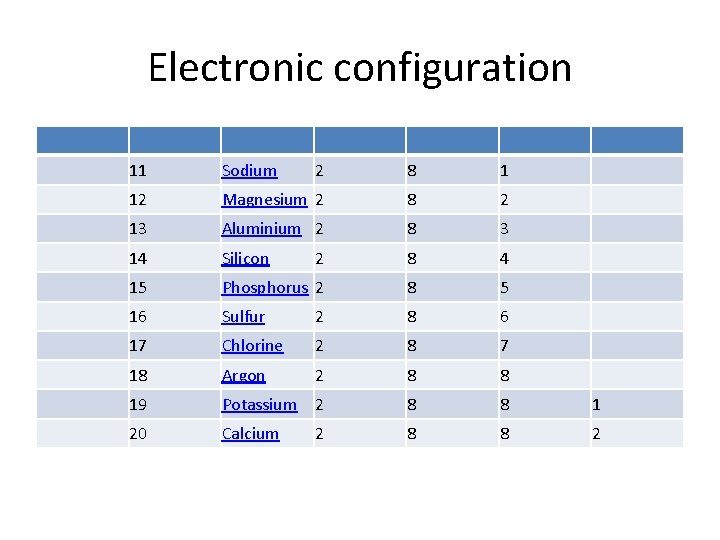
Electronic configuration 11 Sodium 2 8 1 12 Magnesium 2 8 2 13 Aluminium 2 8 3 14 Silicon 2 8 4 15 Phosphorus 2 8 5 16 Sulfur 2 8 6 17 Chlorine 2 8 7 18 Argon 2 8 8 19 Potassium 2 8 8 1 20 Calcium 8 8 2 2

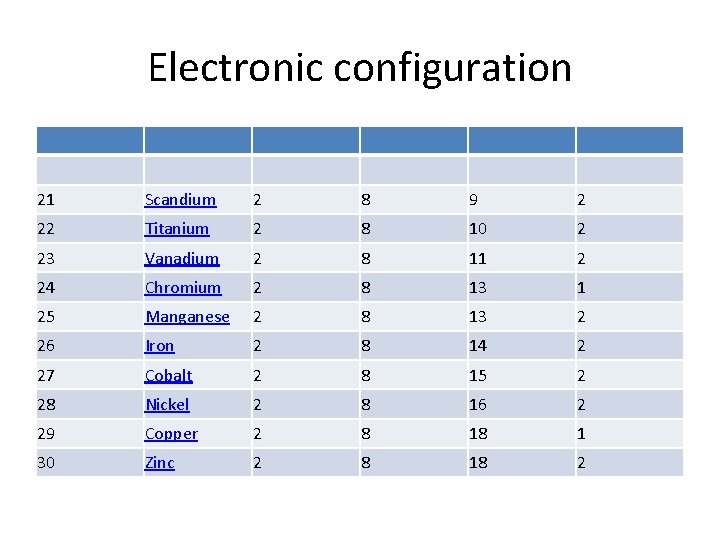
Electronic configuration 21 Scandium 2 8 9 2 22 Titanium 2 8 10 2 23 Vanadium 2 8 11 2 24 Chromium 2 8 13 1 25 Manganese 2 8 13 2 26 Iron 2 8 14 2 27 Cobalt 2 8 15 2 28 Nickel 2 8 16 2 29 Copper 2 8 18 1 30 Zinc 2 8 18 2

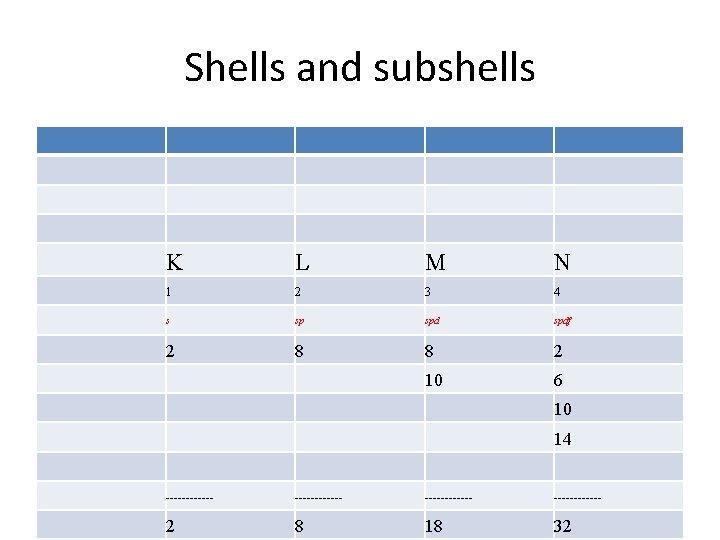
Shells • The shells or energy levels are numbered or lettered, beginning with K. • So K is one, L is two, M is three, N is four, O is five, P is six, and Q is seven. • As the s shells can only have two electrons and the p shells can only have six electrons, the d shells can have only ten electrons and the f shells can have only fourteen electrons.

Shells - Orbitals

Shells • The electron shells are labelled K, L, M, N, O, P, and Q; or 1, 2, 3, 4, 5, 6, and 7; going from innermost shell outwards. • Electrons in outer shells have higher average energy and travel further from the nucleus than those in inner shells, making them more important in determining how the atom reacts chemically and behaves as a conductor, etc, because the pull of the atom's nucleus upon them is weaker and more easily broken.

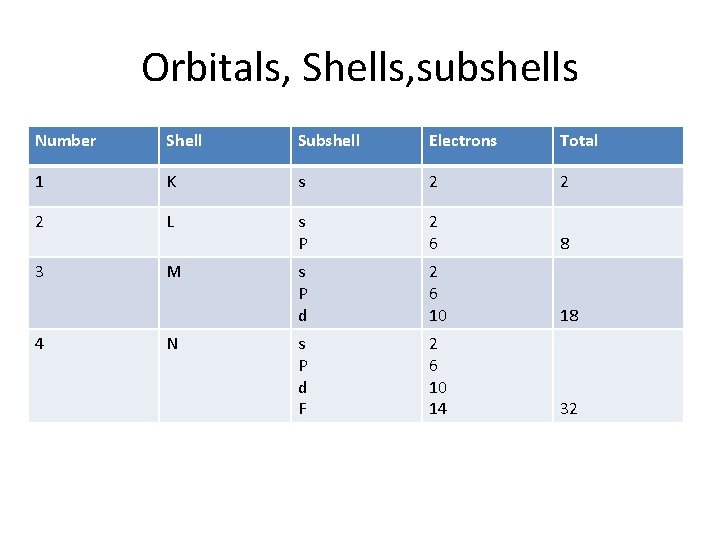
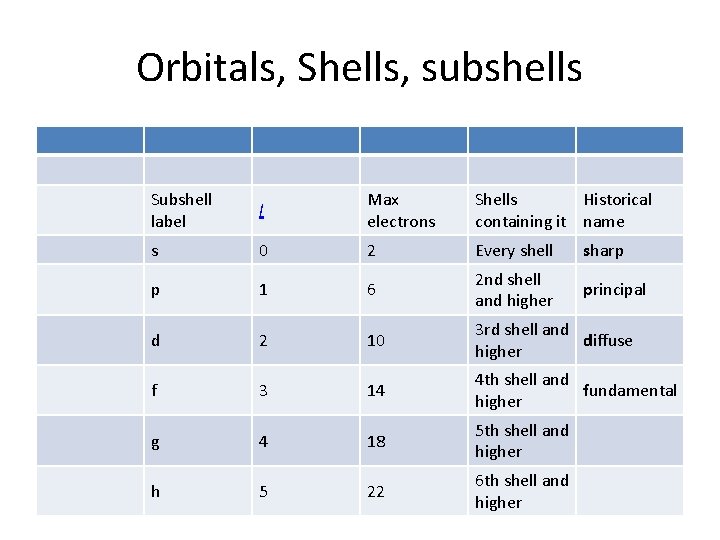
Subshells • Each shell is composed of one or more subshells, which are themselves composed of atomic orbitals. For example, the first (K) shell has one subshell, called "1 s"; the second (L) shell has two subshells, called "2 s" and "2 p"; the third shell has "3 s", "3 p", and "3 d"; and so on. [3] The various possible subshells are shown in the following table:

Orbitals, Shells, subshells Number Shell Subshell Electrons Total 1 K s 2 2 2 L s P 2 6 8 3 M s P d 2 6 10 18 s P d F 2 6 10 14 32 4 N

Orbitals, Shells, subshells Subshell label l Max electrons Shells Historical containing it name s 0 2 Every shell sharp p 1 6 2 nd shell and higher principal d 2 10 3 rd shell and diffuse higher f 3 14 4 th shell and fundamental higher g 4 18 5 th shell and higher h 5 22 6 th shell and higher

Shells and subshells K L M N 1 2 3 4 s sp spdf 2 8 8 2 10 6 10 14 ------------ 2 8 18 32

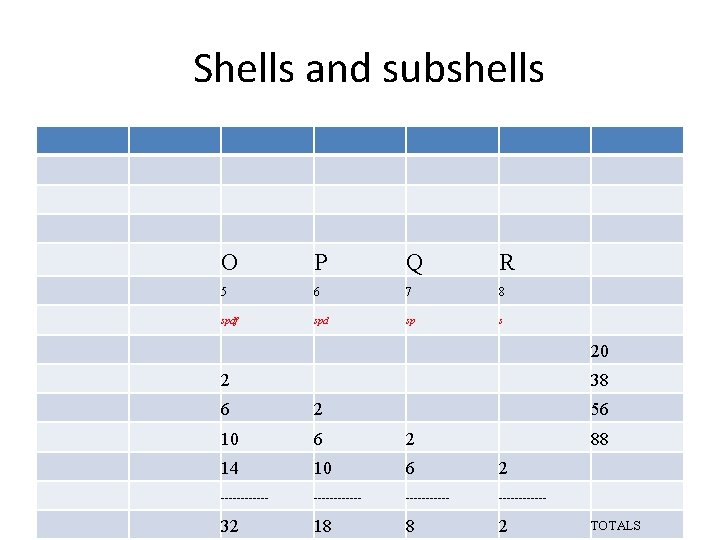
Shells and subshells O P Q R 5 6 7 8 spdf spd sp s 20 2 38 6 2 56 10 6 2 14 10 6 2 ------------ 32 18 8 2 88 TOTALS

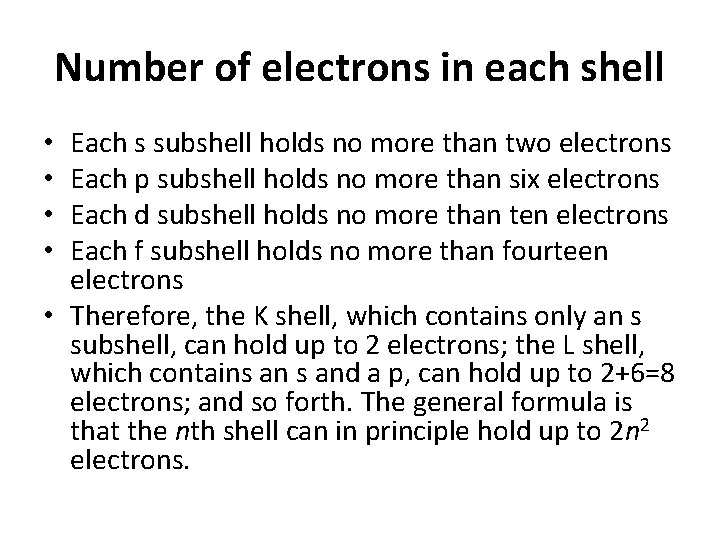
Number of electrons in each shell Each s subshell holds no more than two electrons Each p subshell holds no more than six electrons Each d subshell holds no more than ten electrons Each f subshell holds no more than fourteen electrons • Therefore, the K shell, which contains only an s subshell, can hold up to 2 electrons; the L shell, which contains and a p, can hold up to 2+6=8 electrons; and so forth. The general formula is that the nth shell can in principle hold up to 2 n 2 electrons. • •

Tin: Sn

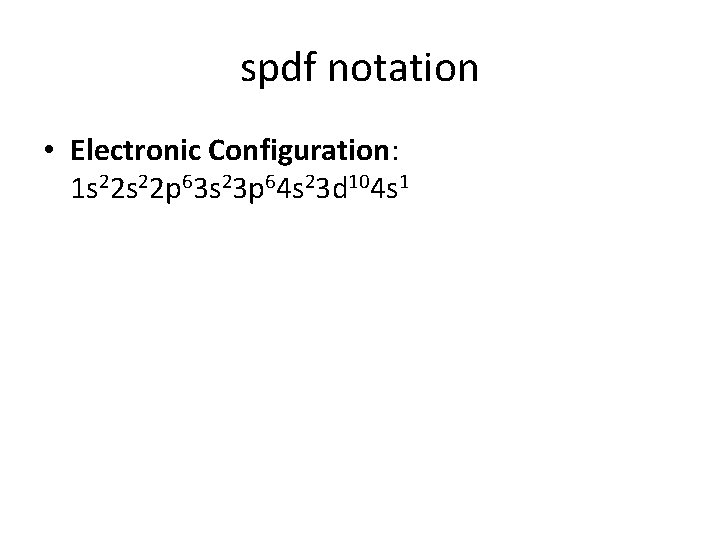
spdf notation • Electronic Configuration: 1 s 22 p 63 s 23 p 64 s 23 d 104 s 1

Spdf notation

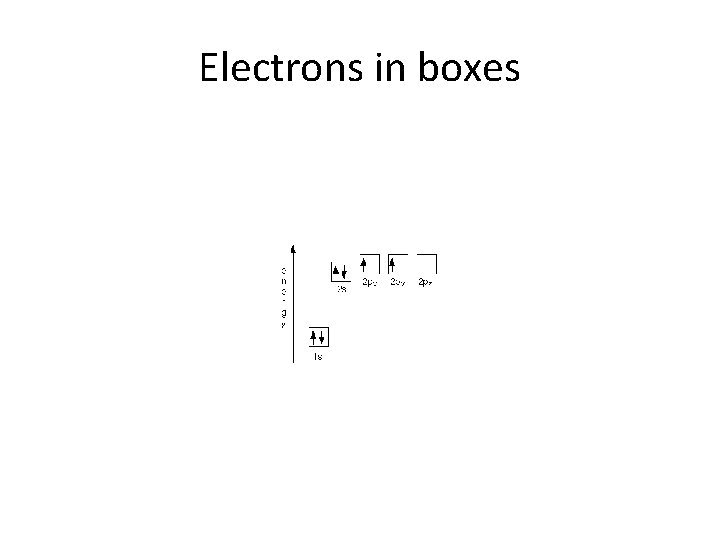
Electrons in boxes

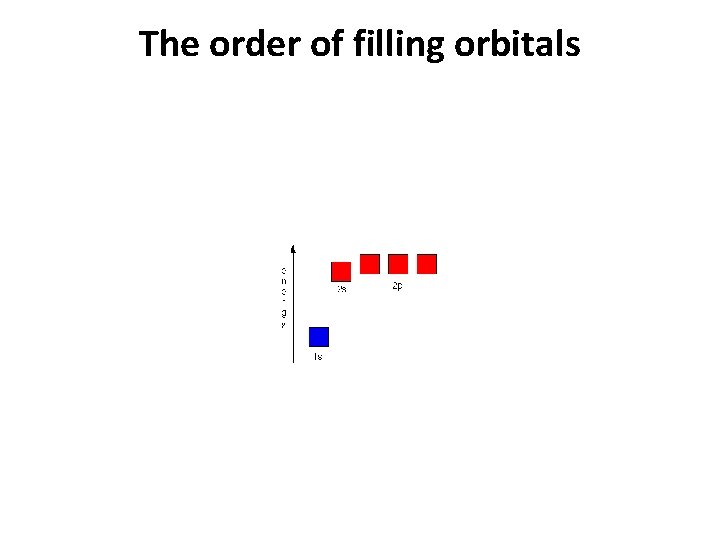
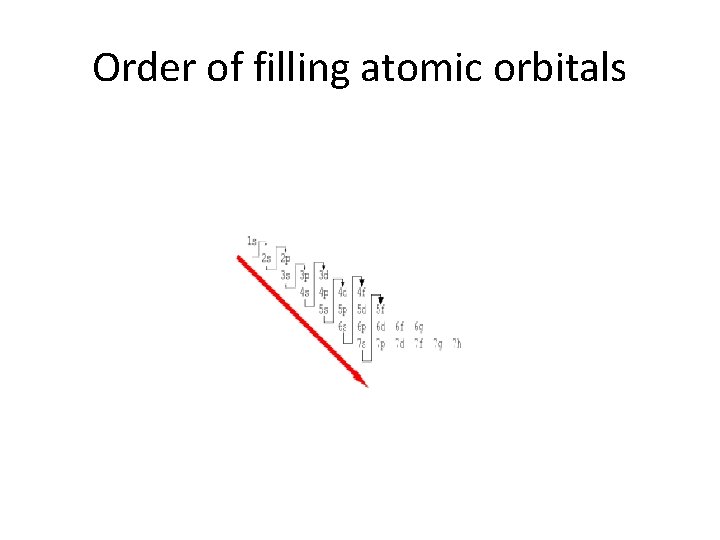
The order of filling orbitals

Orbitals =shells=Energy levels • The circles show energy levels - representing increasing distances from the nucleus. You could straighten the circles out and draw the electronic structure as a simple energy diagram

Energy levels

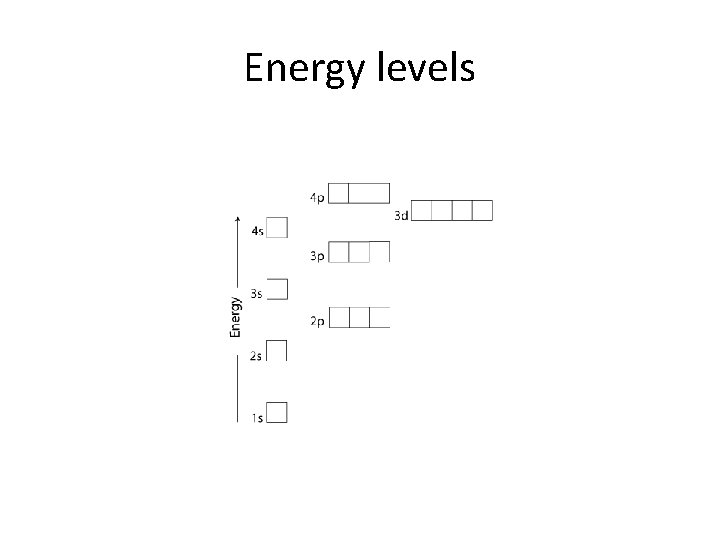
Energy levels

Ground state • The electrons are housed in shells around the nucleus. There is a ranking or hierarchy of the shells, usually with the shells further from the nucleus having a higher energy. • As we consider the electron configuration of atoms, we will be describing the ground state position of the electrons. • Place electrons in lower energy shells. • Start from shell nearest to the nucleus, i. e 1 s 2

Order of filling atomic orbitals

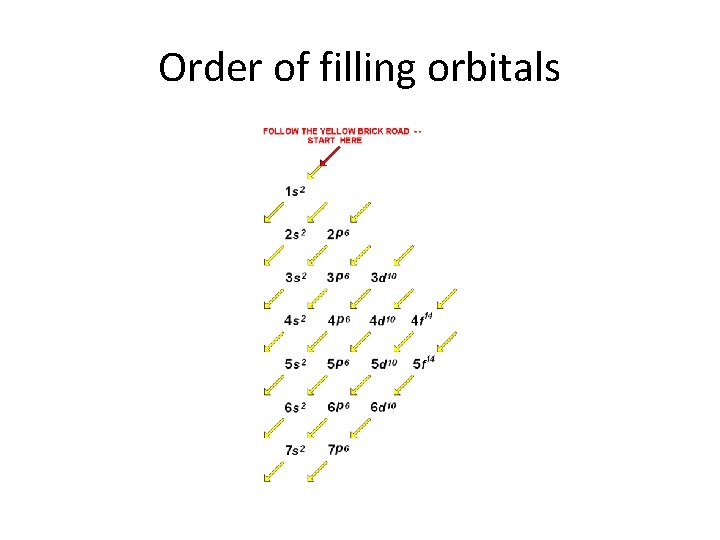
Order of filling orbitals

Ground state • Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. Where there is a choice between orbitals of equal energy, they fill the orbitals singly as far as possible. • The diagram (not to scale) summarises the energies of the various orbitals in the first and second levels. • • Notice that the 2 s orbital has a slightly lower energy than the 2 p orbitals. That means that the 2 s orbital will fill with electrons before the 2 p orbitals. All the 2 p orbitals have exactly the same energy

Quantum • Electrons are able to move from one energy level to another by emission or absorption of a quantum of energy, in the form of a photon. Because of the Pauli exclusion principle, no more than two electrons may exist in a given atomic orbital; therefore an electron may only leap to another orbital if there is a vacancy there. The word comes from the Latin "quantus, " for "how much.

Principal quantum number • An electron shell is the set of atomic orbitals which share the same principal quantum number, n (the number before the letter in the orbital label): hence the 3 s-orbital, the 3 p-orbitals and the 3 d-orbitals all form part of the third shell. • An electron shell can accommodate 2 n 2 electrons, ie the first shell can accommodate 2 electrons, the second shell 8 electrons, the third shell 18 electrons, etc.

The periodic table • The form of the periodic table is closely related to the electron configuration of the atoms of the elements. For example, all the elements of group 2 have an electron configuration of [E] ns 2 (where [E] is an inert gas configuration), and have notable similarities in their chemical properties. • The outermost electron shell is often referred to as the "valence shell" and determines the chemical properties. It should be remembered that the similarities in the chemical properties were remarked more than a century before the idea of electron configuration.

History • The existence of electron shells was first observed experimentally in X-ray absorption studies. Shells were first labeled with the letters K, L, M, N, O, P, and Q. • The origin of this terminology was alphabetic. • Later experiments indicated that the K absorption lines are produced by the innermost electrons. • These letters were later found to correspond to the n-values 1, 2, 3, etc. • K=1 , L =2 , M =3, N = 4, etc.

Quantum theory • The "quantum" theory was proposed more than 90 years ago, and has been confirmed by thousands of experiments. Science and education has failed to clearly describe the energy level concept to almost four generations of citizens. This experiment is an exercise aimed at throwing a little more light on the subject. • Atoms have two kinds of states; a ground state and an excited state. The ground state is the state in which the electrons in the atom are in their lowest energy levels possible (atoms naturally are in the ground state). This means the electrons have the lowest possible values for "n" the principal quantum number.

Spectrum, spectra

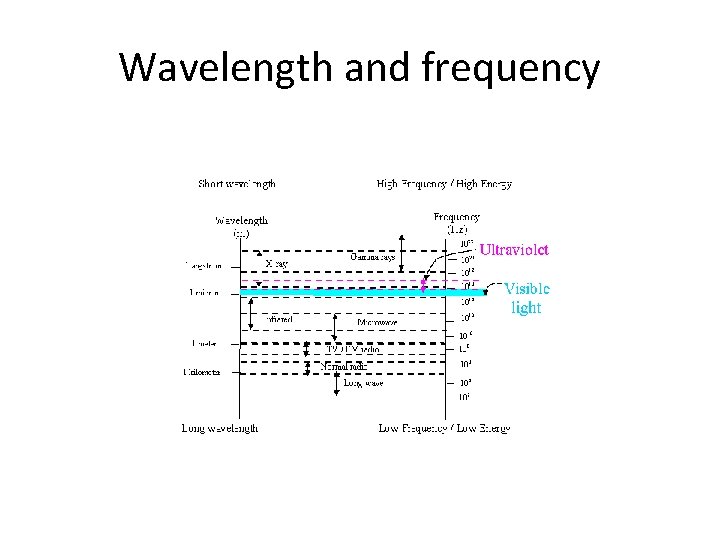
Wavelength and frequency

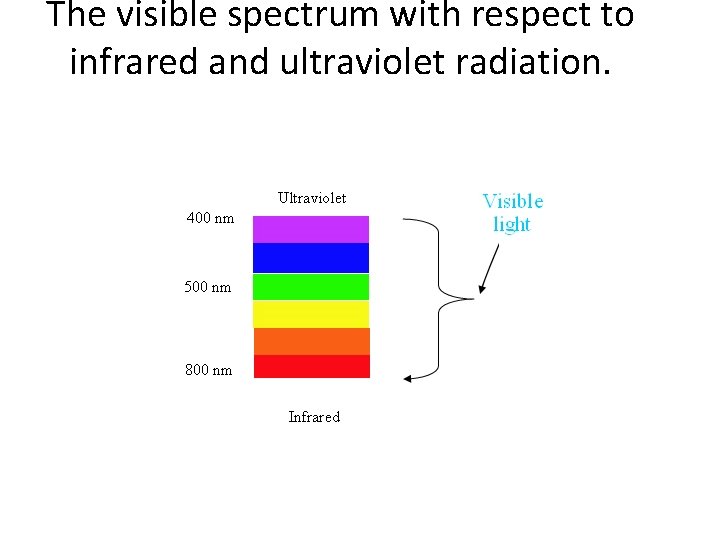
The visible spectrum with respect to infrared and ultraviolet radiation.

UV and visible light • Ultraviolet (200 -400 nm) and visible (400 -800 nm) radiation are found towards the short wavelength, high frequency end of the electromagnetic spectrum. Figure 1 shows the portion of the electromagnetic spectrum where UV-Vis radiation exists.

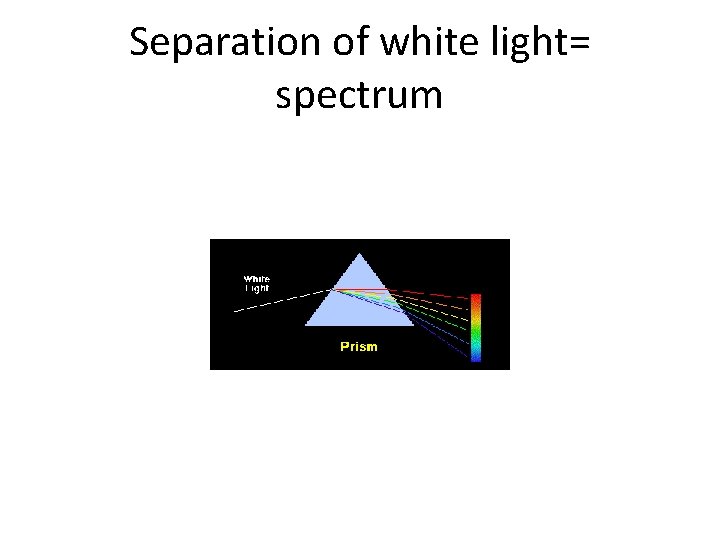
White light • You need to know that white light is the combination of all colors of the spectrum.

Separation of white light= spectrum

Continuous, emission , absorption

Different kinds of spectra • Thus, emission spectra are produced by thin gases in which the atoms do not experience many collisions (because of the low density). The emission lines correspond to photons of discrete energies that are emitted when excited atomic states in the gas make transitions back to lower-lying levels. • A continuum spectrum results when the gas pressures are higher. Generally, solids, liquids, or dense gases emit light at all wavelengths when heated. • An absorption spectrum occurs when light passes through a cold, dilute gas and atoms in the gas absorb at characteristic frequencies; since the re-emitted light is unlikely to be emitted in the same direction as the absorbed photon, this gives rise to dark lines (absence of light) in the spectrum. •

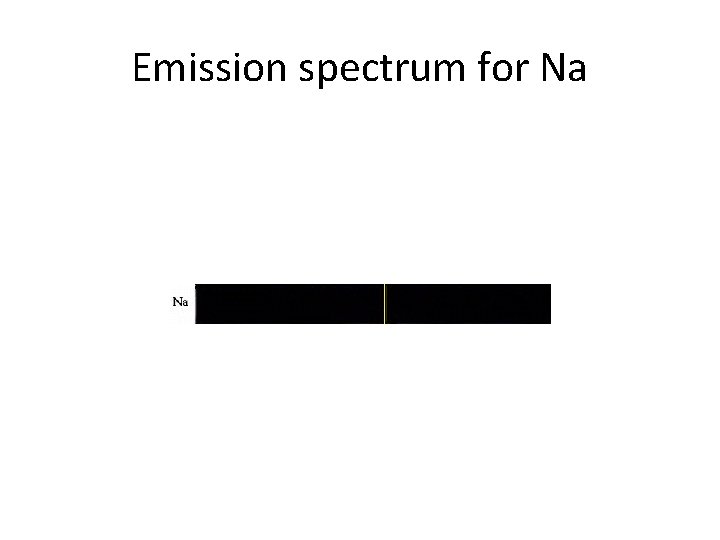
Emission spectrum • An element's 'emission spectrum' is the relative intensity of electromagnetic radiation of each frequency it emits when it is heated (or more generally when it is excited). • When the electrons in the element are excited, they jump to higher energy orbits. As the electrons fall back down, and leave the excited state, energy is re-emitted, the wavelength of which refers to the discrete lines of the emission spectrum. Note, however, that the emission extends over an area of frequencies, an effect called spectral line broadening. This spectrum is caused by hydrogen.

Emission spectrum. . . • The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. One example is identifying the composition of stars by analysing the received light

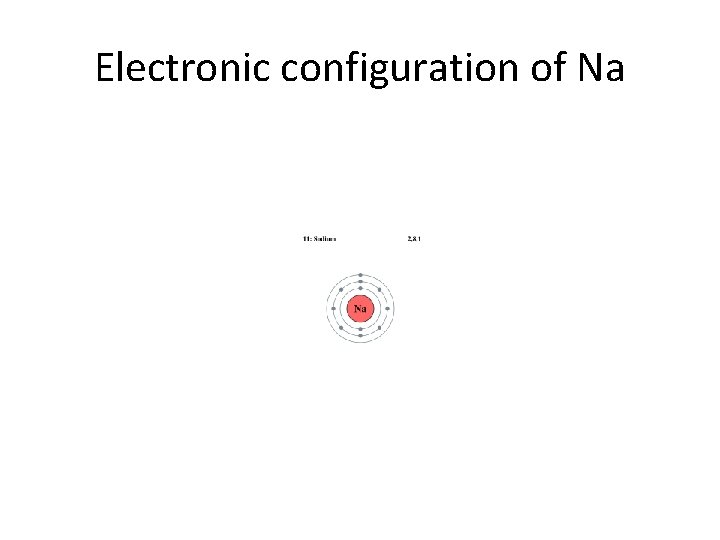
Electronic configuration of Na

Emission spectrum for Na

Absorption spectra

Flame test • A flame test is a procedure used in chemistry to detect the presence of certain metal ions, based on each element's characteristic emission spectrum. The color of flames in general also depends on temperature; see flame color. • The test involves introducing a sample of the element or compound to a hot, non-luminous flame, and observing the color that results. Samples are usually held on a platinum wire cleaned repeatedly with hydrochloric acid to remove traces of previous analytes

Flame test

Flame test

Shells - Orbitals