PRACTICE DRAWING ATOMS DRAWING ATOMS RULES PROTONS Atomic


- Slides: 30

PRACTICE DRAWING ATOMS

DRAWING ATOMS RULES PROTONS = Atomic number ELECTRONS = Atomic number NEUTRONS = mass number – atomic number 1 st level can hold up to 2 electrons 2 nd level can hold up to 8 electrons 3 rd level can hold up to 18 electrons

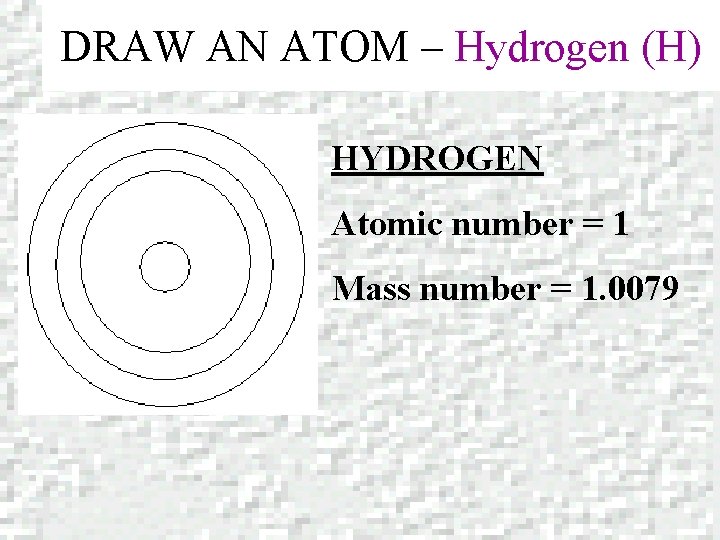
DRAW AN ATOM – Hydrogen (H) HYDROGEN Atomic number = 1 Mass number = 1. 0079

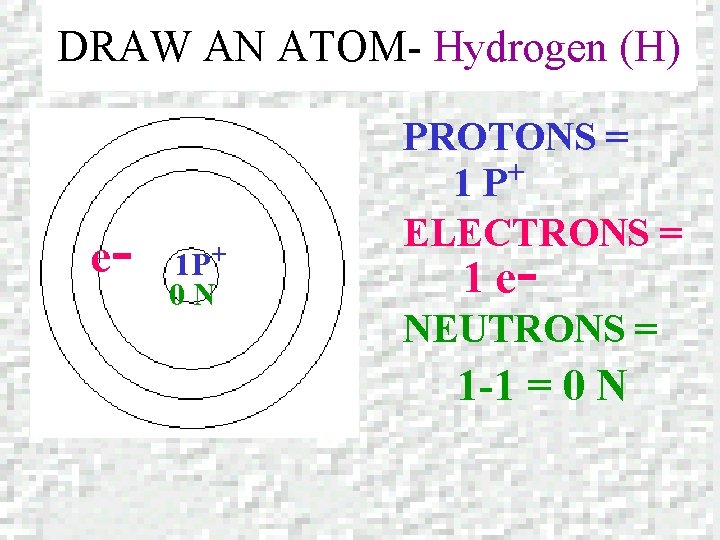
DRAW AN ATOM- Hydrogen (H) e- 1 P+ 0 N PROTONS = + 1 P ELECTRONS = 1 e- NEUTRONS = 1 -1 = 0 N

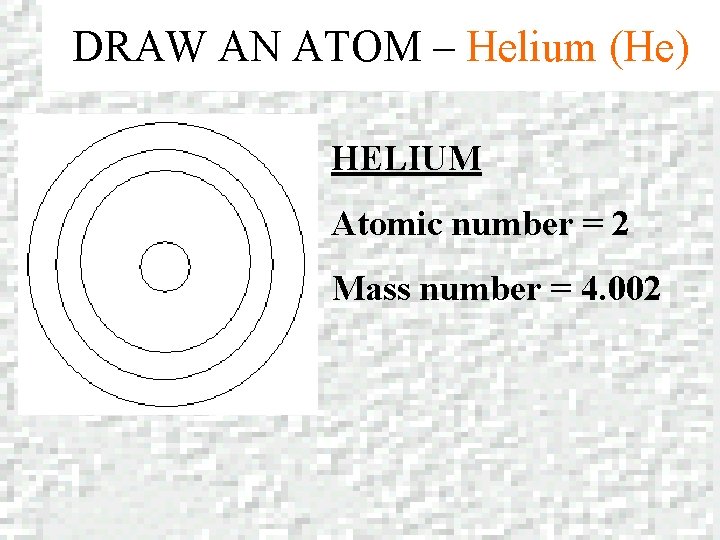
DRAW AN ATOM – Helium (He) HELIUM Atomic number = 2 Mass number = 4. 002

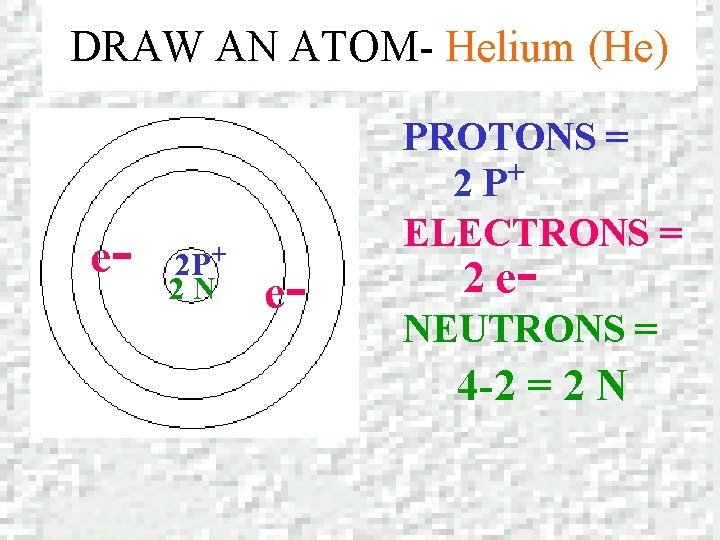
DRAW AN ATOM- Helium (He) e- 2 P+ 2 N e- PROTONS = + 2 P ELECTRONS = 2 e- NEUTRONS = 4 -2 = 2 N

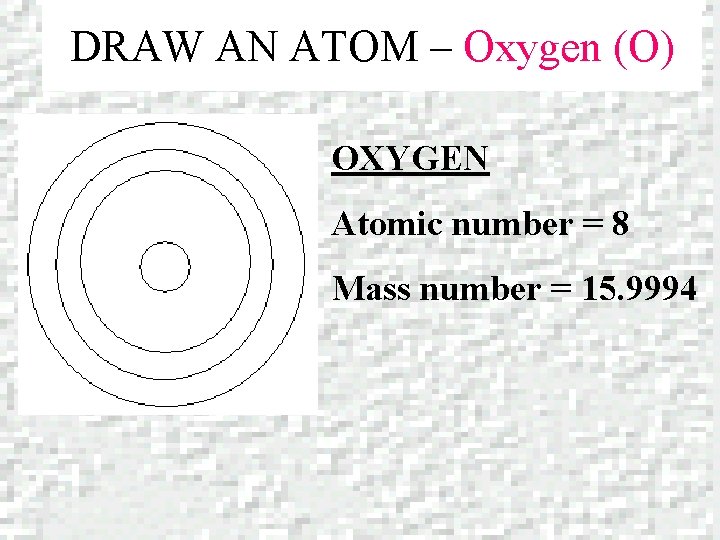
DRAW AN ATOM – Oxygen (O) OXYGEN Atomic number = 8 Mass number = 15. 9994

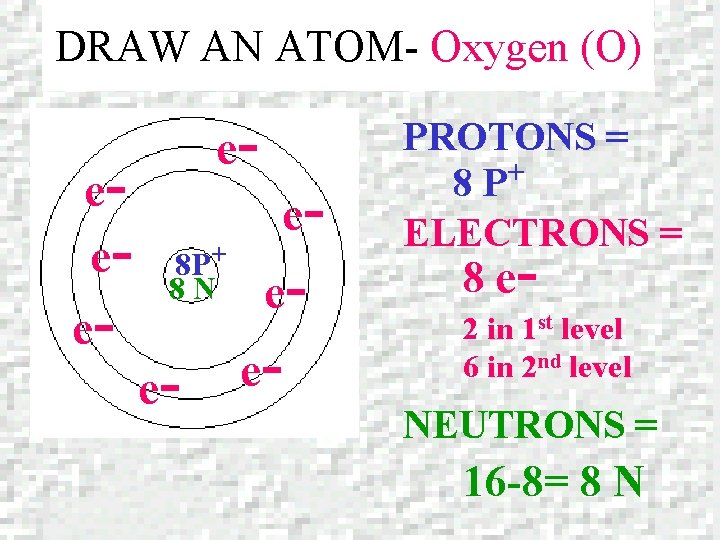
DRAW AN ATOM- Oxygen (O) ee- e- e 8 P+ 8 N e- e- PROTONS = + 8 P ELECTRONS = 8 e- 2 in 1 st level 6 in 2 nd level NEUTRONS = 16 -8= 8 N

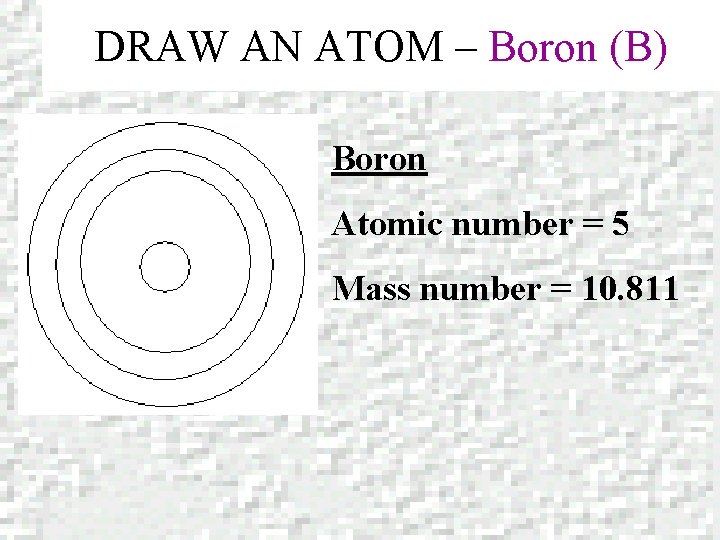
DRAW AN ATOM – Boron (B) Boron Atomic number = 5 Mass number = 10. 811

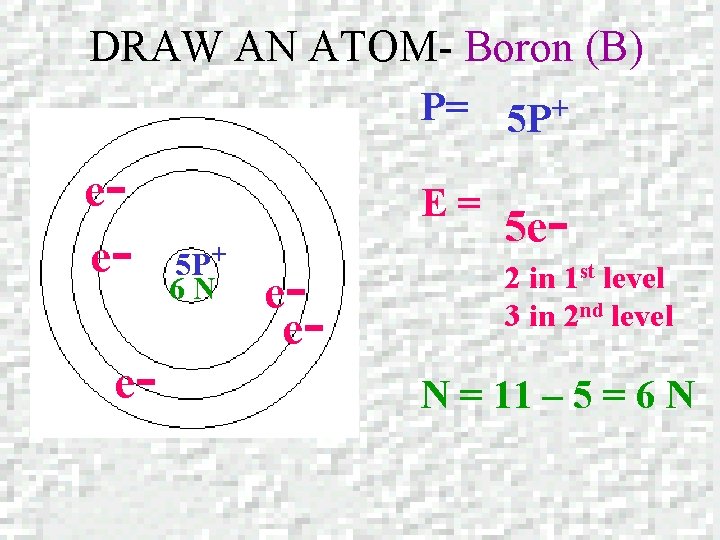
DRAW AN ATOM- Boron (B) P= 5 P+ eee- E= 5 P+ 6 N ee- 5 e 2 in 1 st level 3 in 2 nd level N = 11 – 5 = 6 N

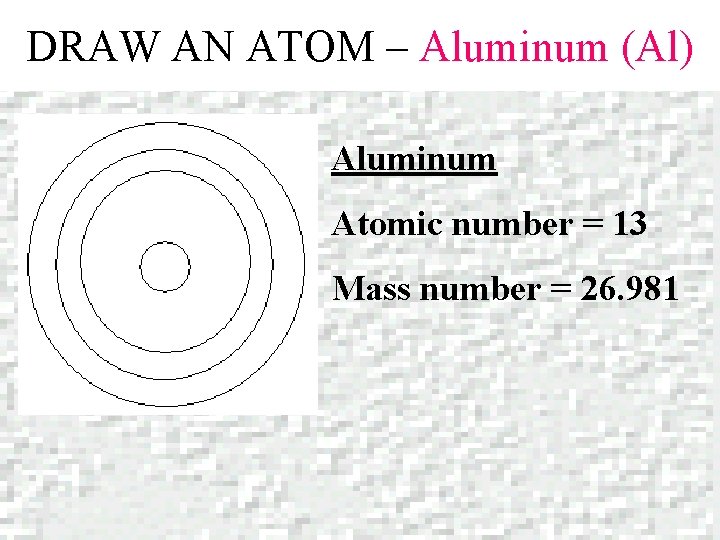
DRAW AN ATOM – Aluminum (Al) Aluminum Atomic number = 13 Mass number = 26. 981

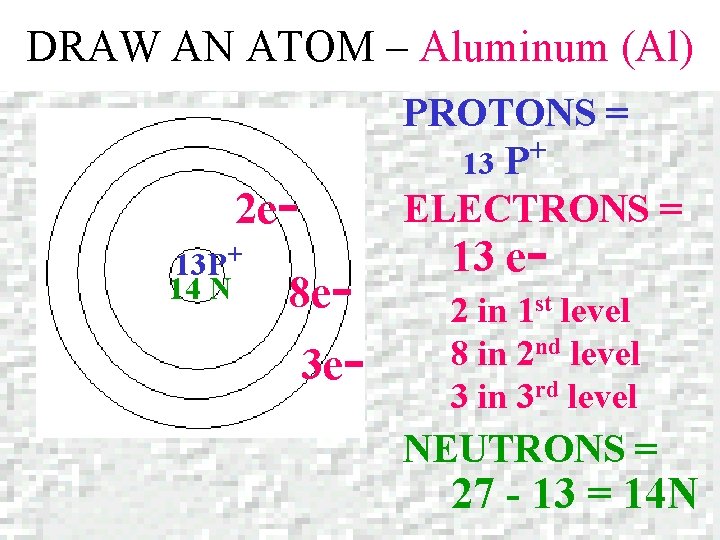
DRAW AN ATOM – Aluminum (Al) PROTONS = + 13 P ELECTRONS = 2 e 13 P+ 14 N 8 e- 3 e- 13 e- 2 in 1 st level 8 in 2 nd level 3 in 3 rd level NEUTRONS = 27 - 13 = 14 N

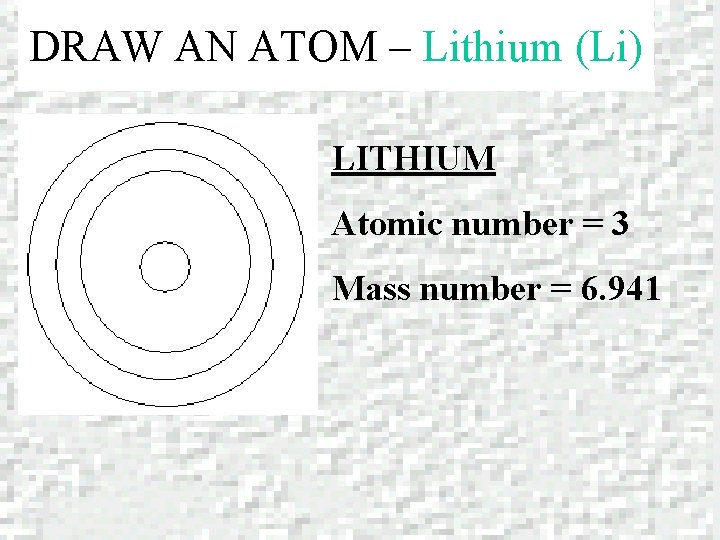
DRAW AN ATOM – Lithium (Li) LITHIUM Atomic number = 3 Mass number = 6. 941

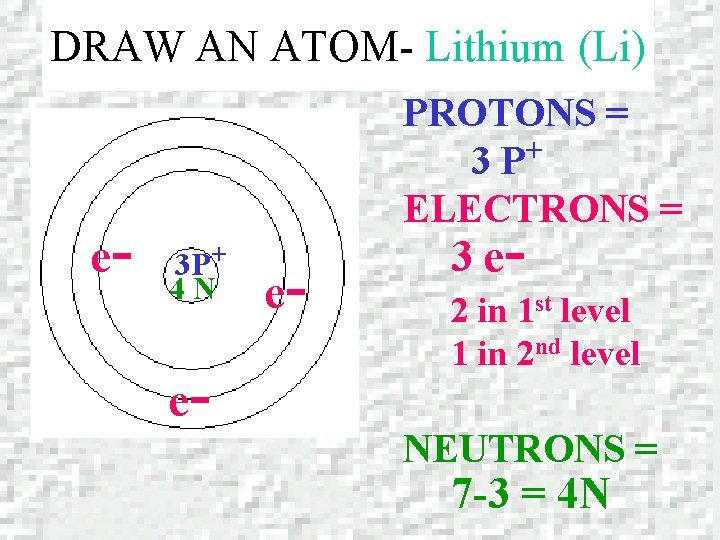
DRAW AN ATOM- Lithium (Li) e- PROTONS = + 3 P ELECTRONS = 3 P+ 4 N e- e- 3 e- 2 in 1 st level 1 in 2 nd level NEUTRONS = 7 -3 = 4 N

DRAW AN ATOM – Nitrogen (N) NITROGEN Atomic number = 7 Mass number = 14. 0067

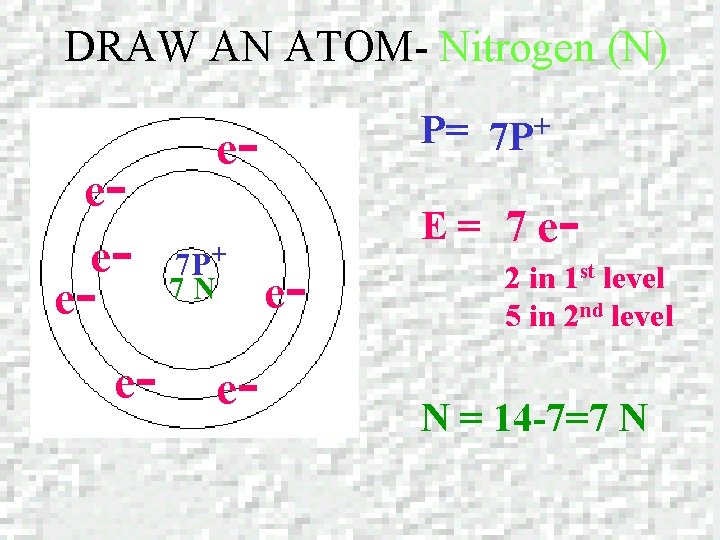
DRAW AN ATOM- Nitrogen (N) ee- e- e- e 7 P+ 7 N e- P= 7 P+ e- E= 7 e - 2 in 1 st level 5 in 2 nd level N = 14 -7=7 N

DRAW AN ATOM – Phosphorus (P) Phosphorus Atomic number = 15 Mass number = 30. 973

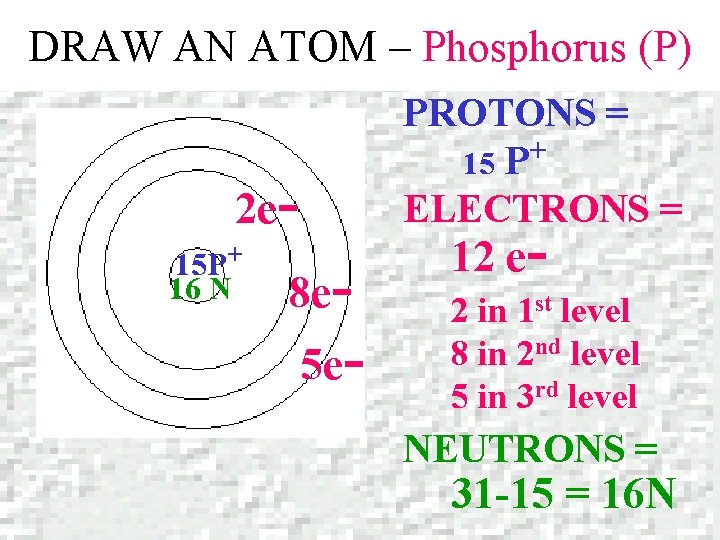
DRAW AN ATOM – Phosphorus (P) PROTONS = + 15 P ELECTRONS = 2 e 15 P+ 16 N 8 e- 5 e- 12 e- 2 in 1 st level 8 in 2 nd level 5 in 3 rd level NEUTRONS = 31 -15 = 16 N

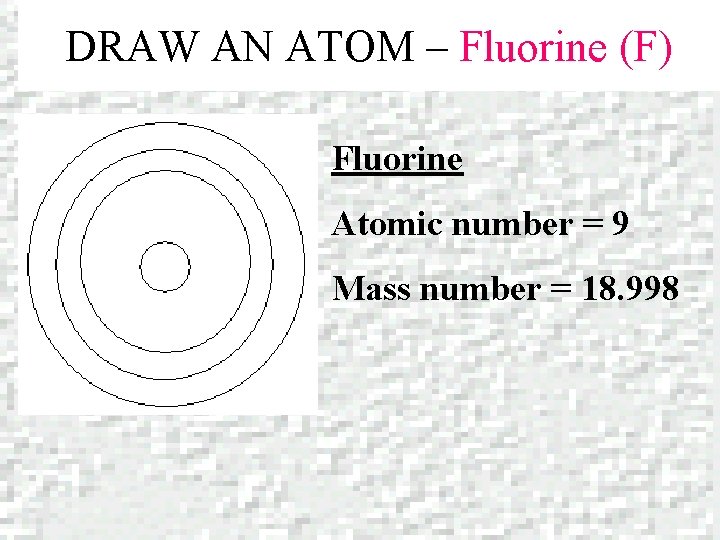
DRAW AN ATOM – Fluorine (F) Fluorine Atomic number = 9 Mass number = 18. 998

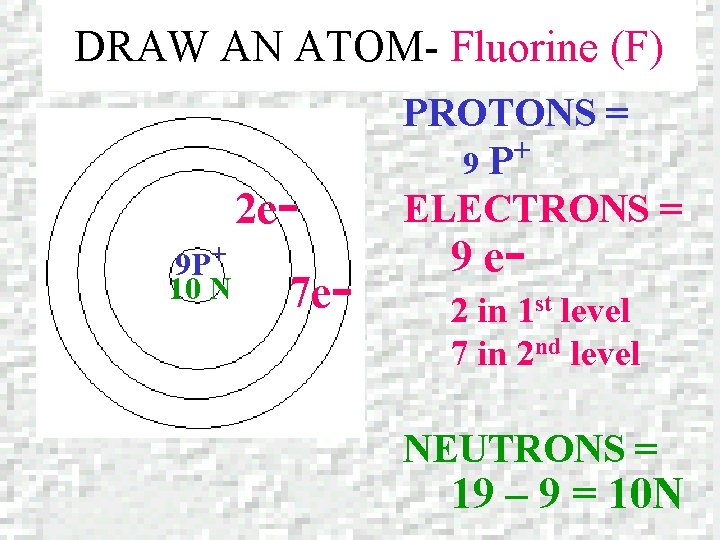
DRAW AN ATOM- Fluorine (F) 2 e 9 P+ 10 N 7 e- PROTONS = + 9 P ELECTRONS = 9 e- 2 in 1 st level 7 in 2 nd level NEUTRONS = 19 – 9 = 10 N

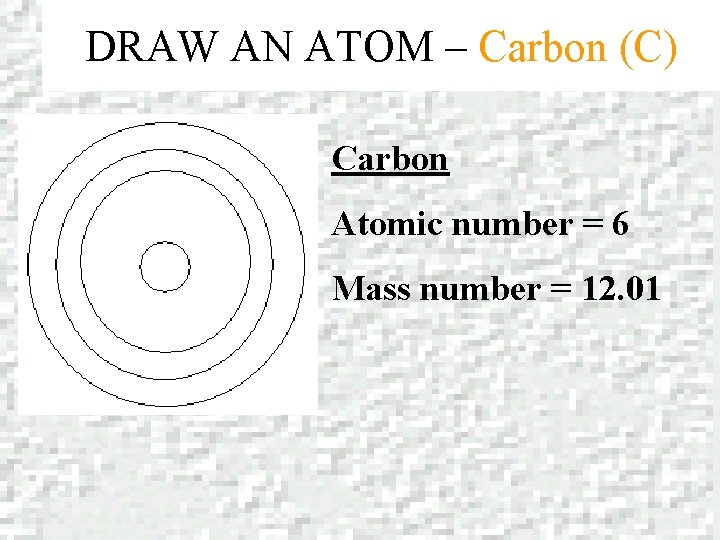
DRAW AN ATOM – Carbon (C) Carbon Atomic number = 6 Mass number = 12. 01

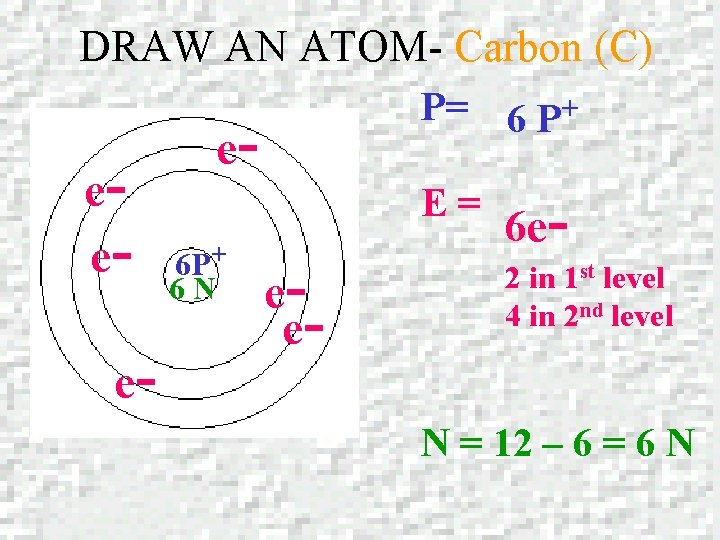
DRAW AN ATOM- Carbon (C) eee- P= 6 P+ e- E= 6 P+ 6 N ee- 6 e 2 in 1 st level 4 in 2 nd level N = 12 – 6 = 6 N

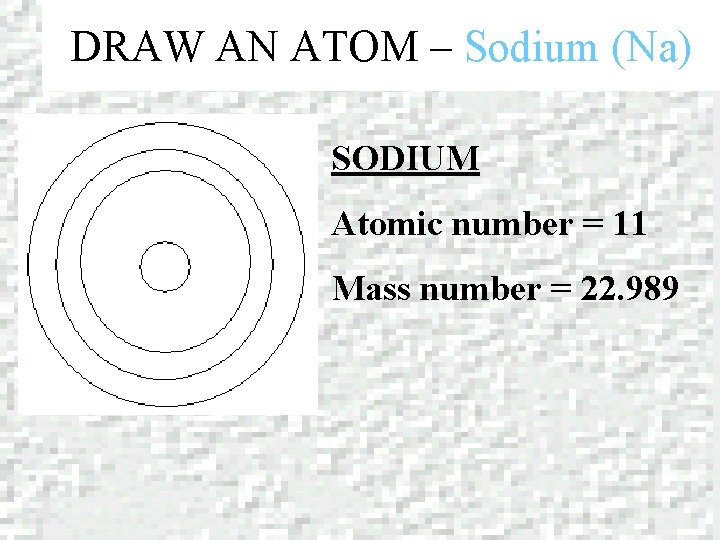
DRAW AN ATOM – Sodium (Na) SODIUM Atomic number = 11 Mass number = 22. 989

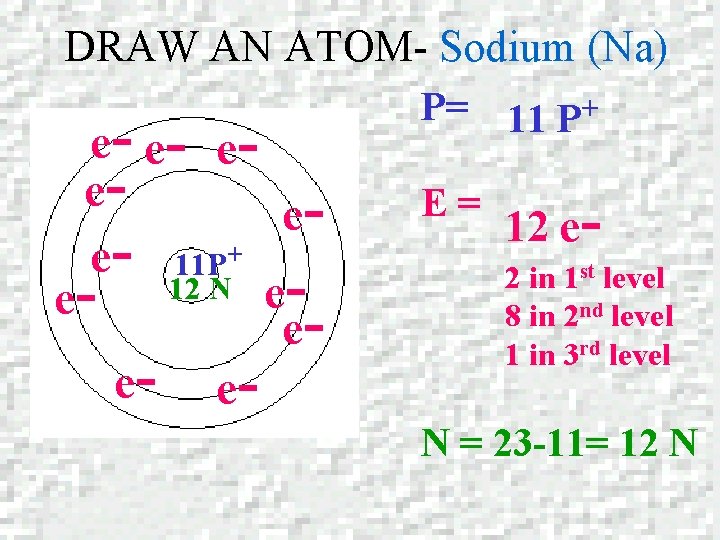
DRAW AN ATOM- Sodium (Na) e- e- eeee- 11 P+ 12 N eeee- e- P= 11 P+ E= 12 e 2 in 1 st level 8 in 2 nd level 1 in 3 rd level N = 23 -11= 12 N

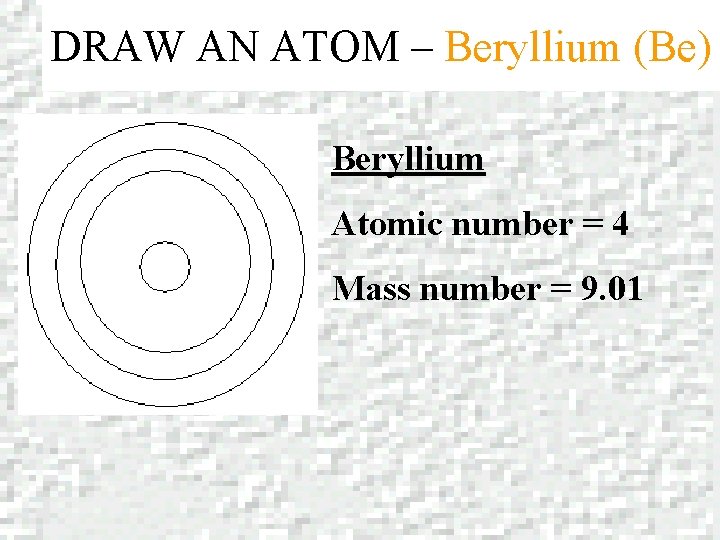
DRAW AN ATOM – Beryllium (Be) Beryllium Atomic number = 4 Mass number = 9. 01

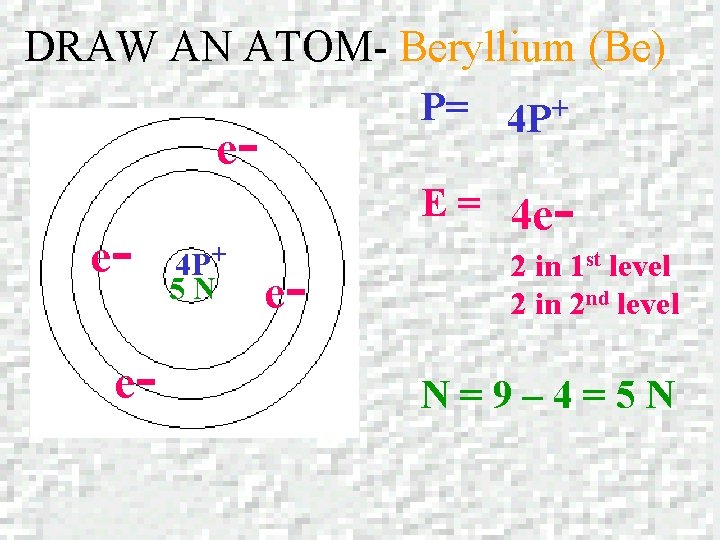
DRAW AN ATOM- Beryllium (Be) P= 4 P+ eee- E = 4 e 4 P+ 5 N e- - 2 in 1 st level 2 in 2 nd level N=9– 4=5 N

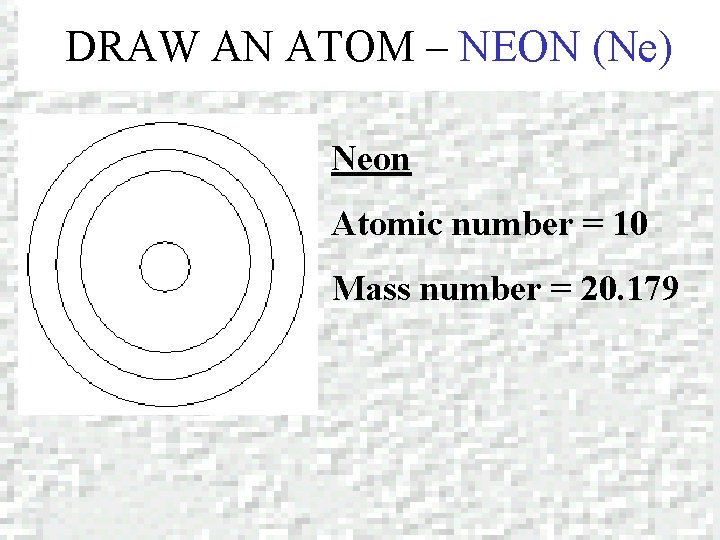
DRAW AN ATOM – NEON (Ne) Neon Atomic number = 10 Mass number = 20. 179

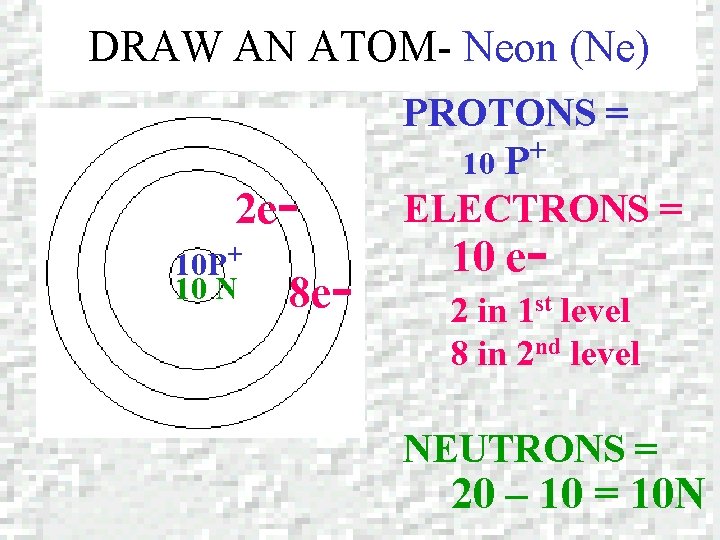
DRAW AN ATOM- Neon (Ne) 2 e 10 P+ 10 N 8 e- PROTONS = + 10 P ELECTRONS = 10 e- 2 in 1 st level 8 in 2 nd level NEUTRONS = 20 – 10 = 10 N

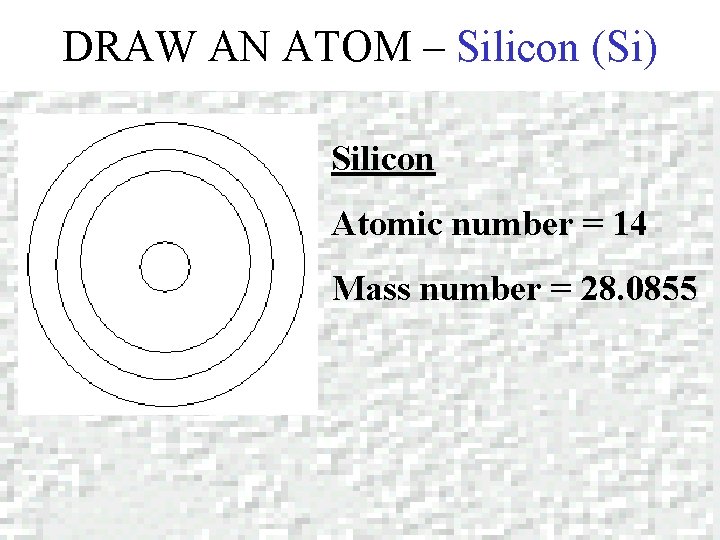
DRAW AN ATOM – Silicon (Si) Silicon Atomic number = 14 Mass number = 28. 0855

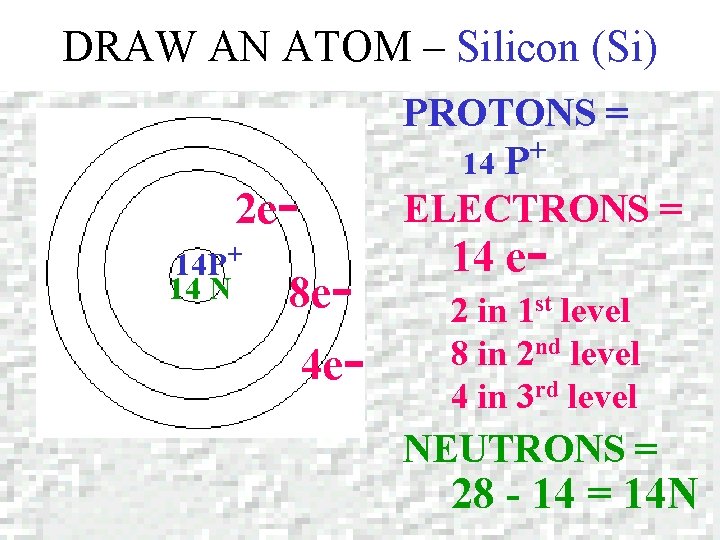
DRAW AN ATOM – Silicon (Si) PROTONS = + 14 P ELECTRONS = 2 e 14 P+ 14 N 8 e- 4 e- 14 e- 2 in 1 st level 8 in 2 nd level 4 in 3 rd level NEUTRONS = 28 - 14 = 14 N