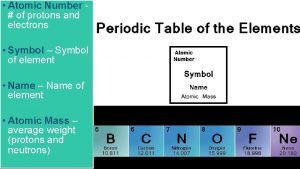
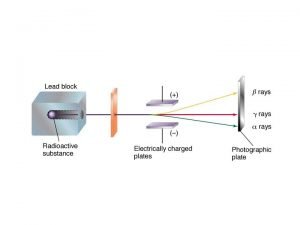
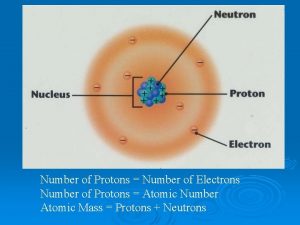
Number of Protons Atomic Number Number of Protons

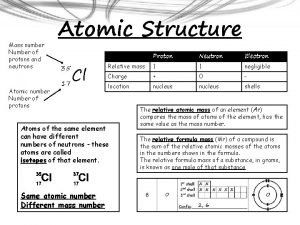
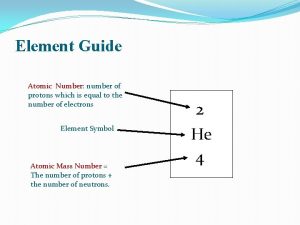
Number of Protons Atomic Number

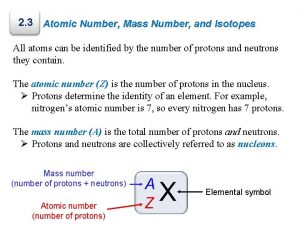
Number of Protons + Neutrons Mass Number

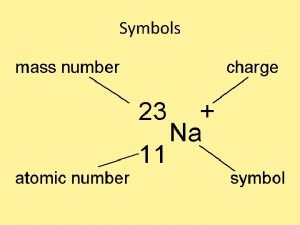
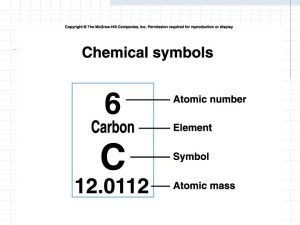
Left Superscript = mass number 12 C 6

Left Subscript = atomic number 12 C 6

35 80 Br 35 Atomic Number = ?
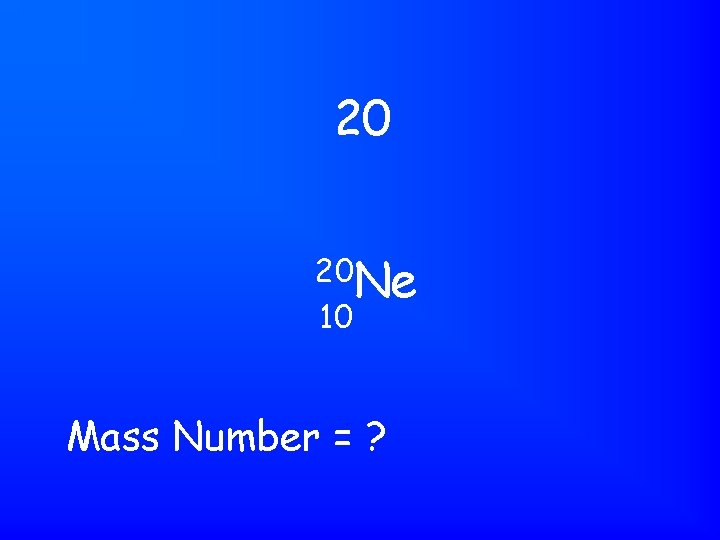
20 20 Ne 10 Mass Number = ?

27 27 Al 13 Mass Number = ?

20 40 Ca 20 Atomic Number = ?
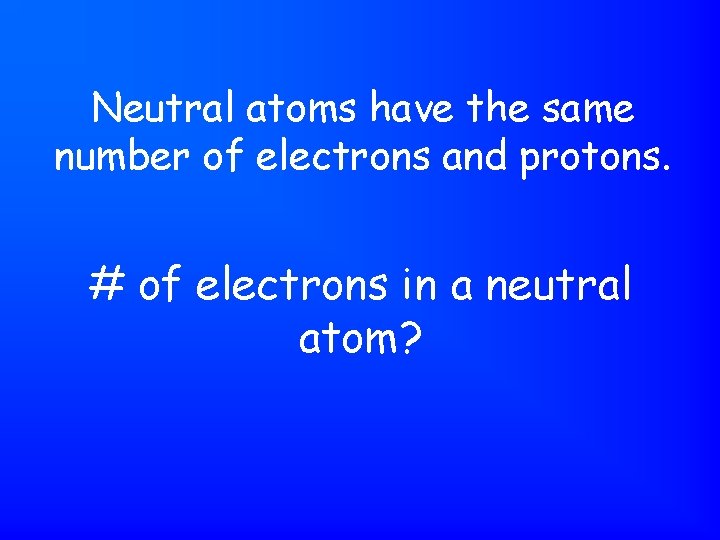
Neutral atoms have the same number of electrons and protons. # of electrons in a neutral atom?
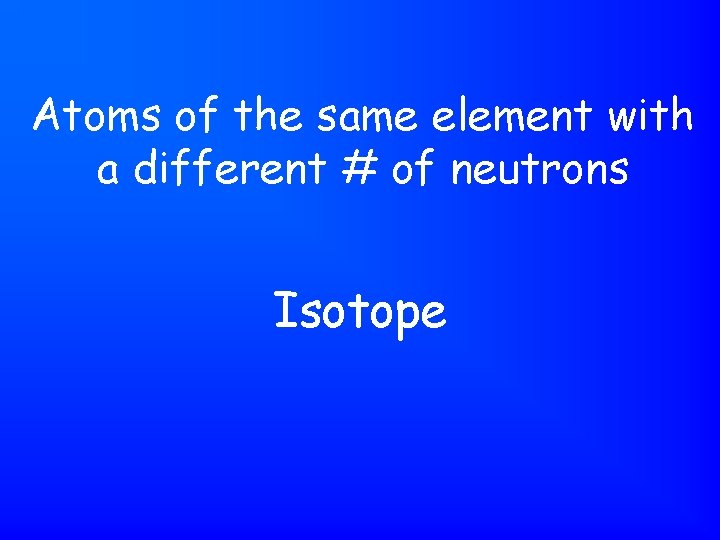
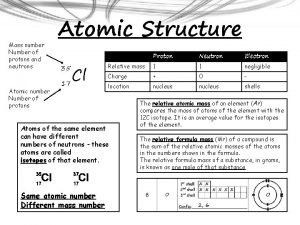
Atoms of the same element with a different # of neutrons Isotope
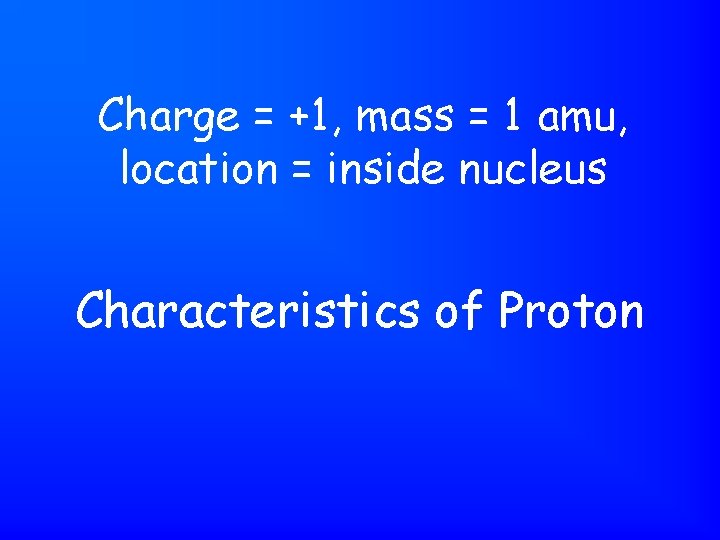
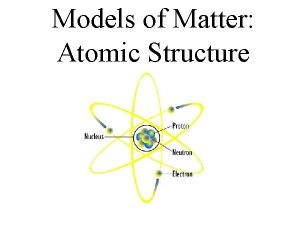
Charge = +1, mass = 1 amu, location = inside nucleus Characteristics of Proton

Charge = 0, mass = 1 amu, location = inside nucleus Characteristics of Neutron

Charge = -1, mass = 1/1836 amu or 0. 0005 amu, location = outside nucleus Characteristics of Electron

An atom that has gained or lost electrons & so carries charge Ion

An atom that has LOST electrons Positive Ion

An atom that has GAINED electrons Negative Ion
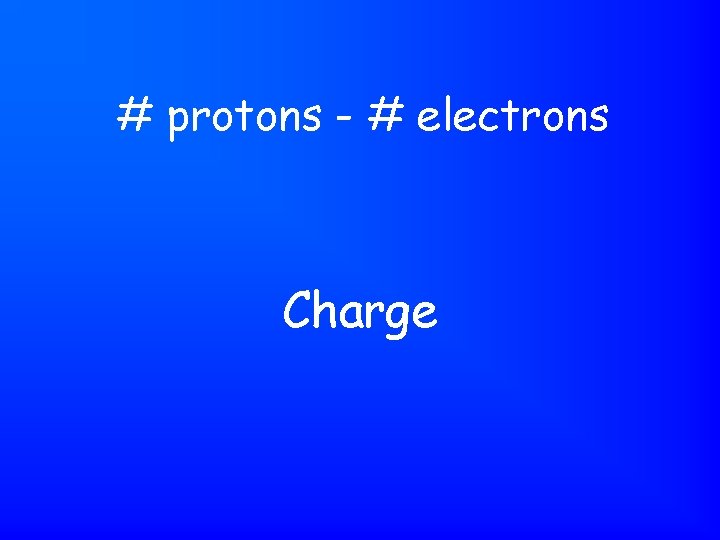
# protons - # electrons Charge
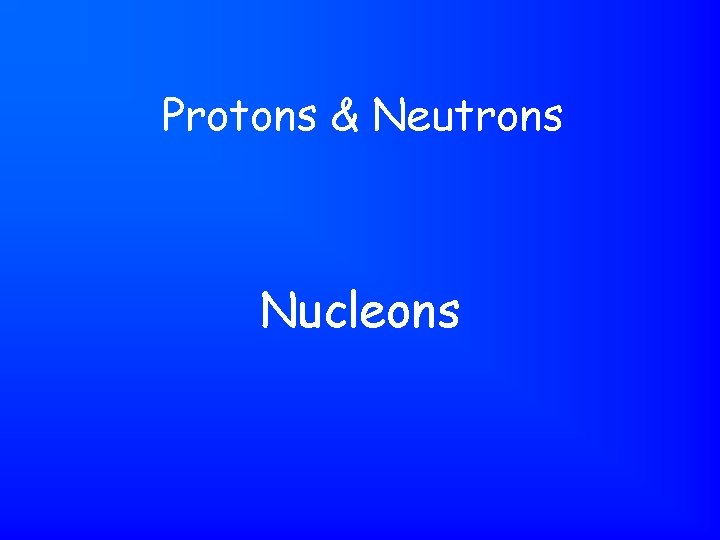
Protons & Neutrons Nucleons

Smallest bit of an element that retains the properties of the element. atom

Mass number – atomic number # of neutrons
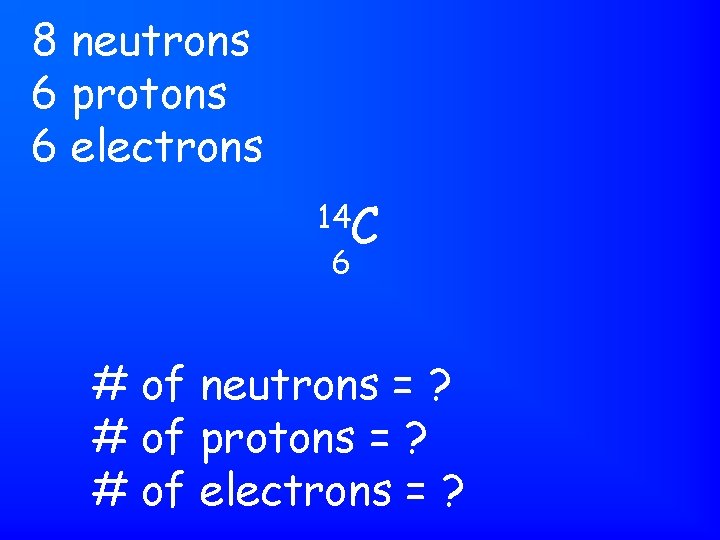
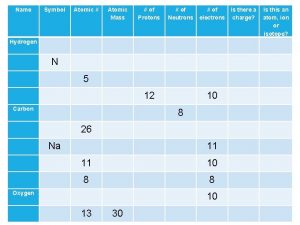
8 neutrons 6 protons 6 electrons 14 C 6 # of neutrons = ? # of protons = ? # of electrons = ?
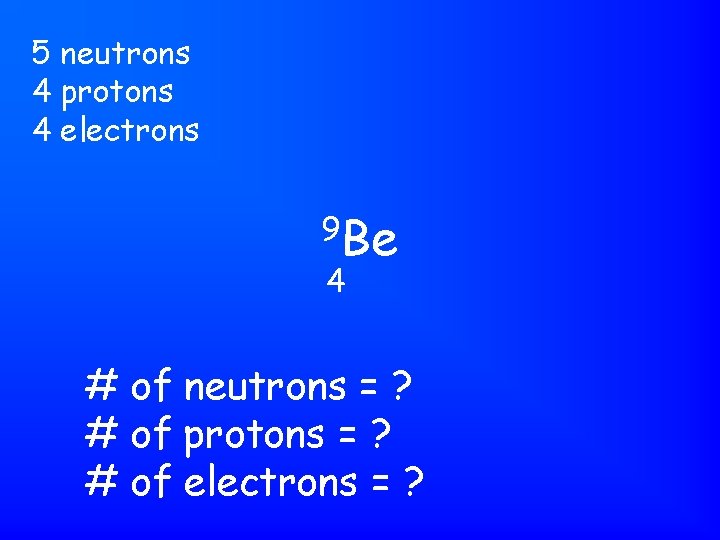
5 neutrons 4 protons 4 electrons 9 Be 4 # of neutrons = ? # of protons = ? # of electrons = ?

22 neutrons 18 protons 18 electrons 40 Ar 18 # of neutrons = ? # of protons = ? # of electrons = ?
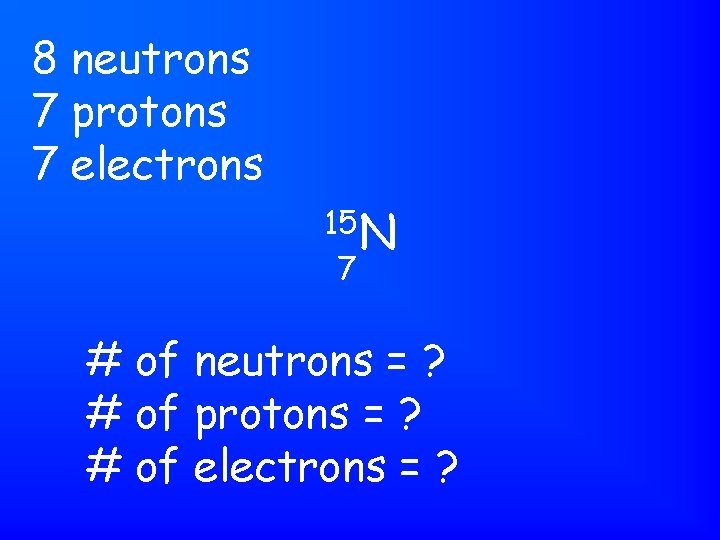
8 neutrons 7 protons 7 electrons 15 N 7 # of neutrons = ? # of protons = ? # of electrons = ?

Right superscript = charge 2+ 24 Mg 12
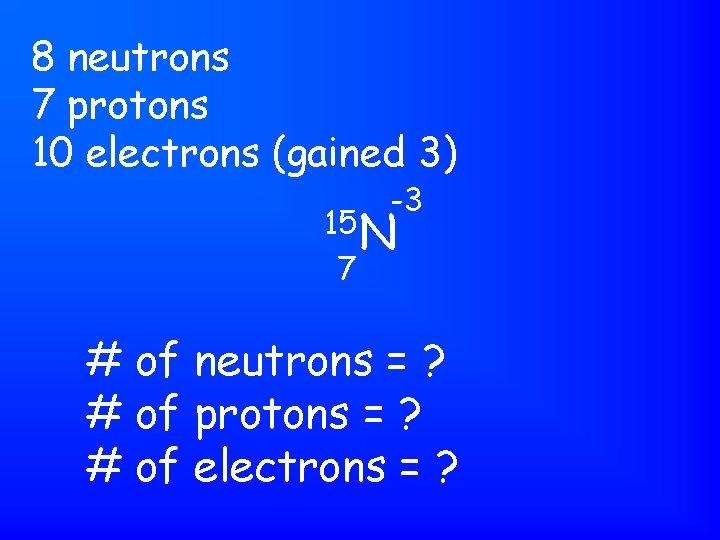
8 neutrons 7 protons 10 electrons (gained 3) -3 15 N 7 # of neutrons = ? # of protons = ? # of electrons = ?

10 neutrons 9 protons 10 electrons (gained 1) -1 19 F 9 # of neutrons = ? # of protons = ? # of electrons = ?
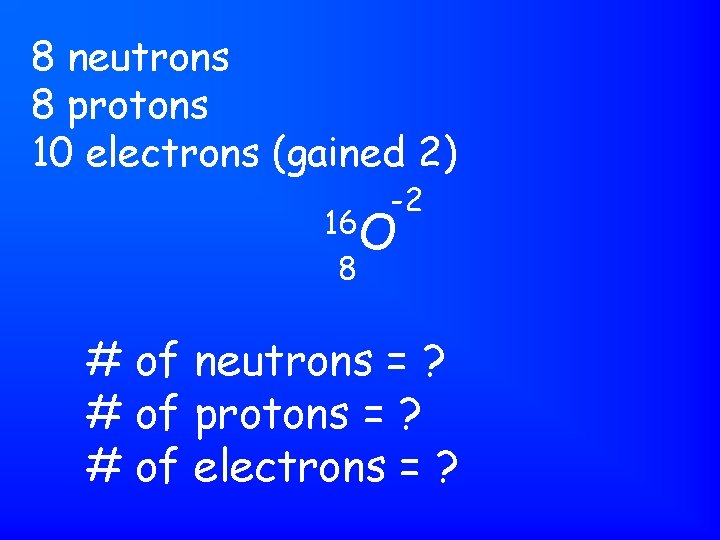
8 neutrons 8 protons 10 electrons (gained 2) -2 16 O 8 # of neutrons = ? # of protons = ? # of electrons = ?
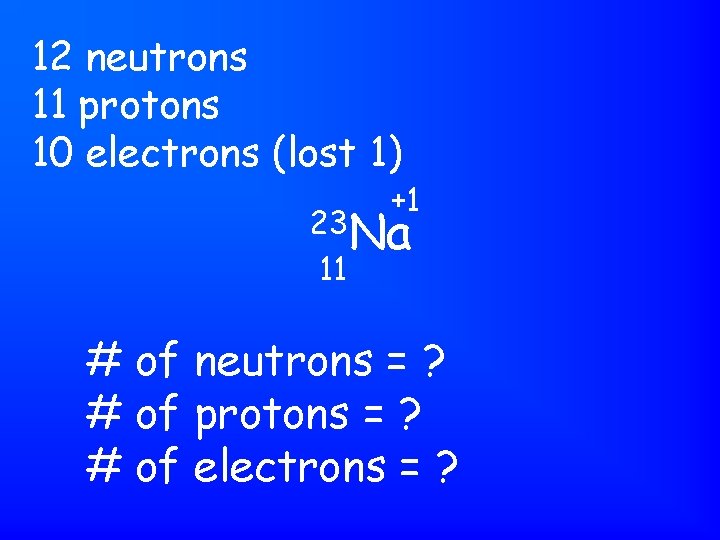
12 neutrons 11 protons 10 electrons (lost 1) +1 23 Na 11 # of neutrons = ? # of protons = ? # of electrons = ?
12 neutrons 12 protons 10 electrons (lost 2) +2 24 Mg 12 # of neutrons = ? # of protons = ? # of electrons = ?
14 neutrons 13 protons 10 electrons (lost 3) +3 27 Al 13 # of neutrons = ? # of protons = ? # of electrons = ?

• Charge on the nucleus only. Does not include the electrons. • Always positive. • Equals the number of protons. Nuclear Charge

Positive ion Cation

Negative ion Anion

Billiard Ball Model Dalton’s Model

Atoms are hard, round, indivisible. They are solid & uniform throughout. Dalton’s model

Plum Pudding Model Thomson’s Model - + + -+ -

Atoms are divisible. They are solid but NOT uniform throughout. They contain + and - charges. The – charges are little particles. Thomson’s model
Nuclear Model Rutherford’s Model - + -

Atoms are divisible. Atoms are NOT solid. Atoms are NOT uniform. They contain + and - charges. Most of the mass and all of the + charge is concentrated in the nucleus of the atom. Most of the volume is empty space. Rutherford’s model

Shot α particles at gold foil. Most went through, so most of the atom is empty space. Some deflected back by small dense positive nucleus. Rutherford’s Experiment
Planetary Model Bohr’s Model
The mass of the entire atom: includes protons, neutrons, electrons. Expressed relative to the mass of a C-12 atomic mass

1 atomic mass unit 1/12 the mass of a C-12 atom. or The C-12 atom has a mass of 12. 000. . . atomic mass units. atomic mass unit

The weighted average of the masses of the naturally occurring isotopes of an element. Average atomic mass

1) Convert % abundance to decimal format. 2) Multiply abundance factor by appropriate mass. 3) Sum Average atomic mass

1) Final answer must be between the highest & lowest masses. 2) Final answer will be closest to mass of most abundant isotope. Quick check on average atomic mass calculation.
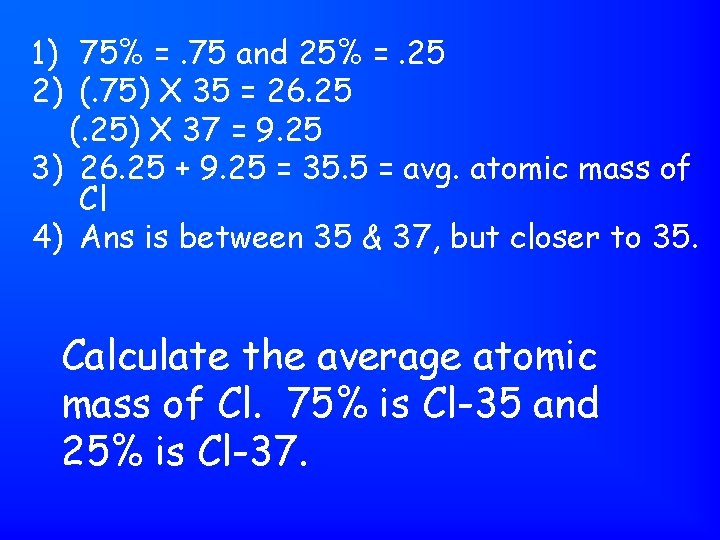
1) 75% =. 75 and 25% =. 25 2) (. 75) X 35 = 26. 25 (. 25) X 37 = 9. 25 3) 26. 25 + 9. 25 = 35. 5 = avg. atomic mass of Cl 4) Ans is between 35 & 37, but closer to 35. Calculate the average atomic mass of Cl. 75% is Cl-35 and 25% is Cl-37.
- Slides: 48