Chapter 4 Atomic Structure Electronic Structure of Atoms








![Electron Configuration ØNa: 1 s 2 2 p 6 3 s 1 ØNa: [Ne] Electron Configuration ØNa: 1 s 2 2 p 6 3 s 1 ØNa: [Ne]](https://slidetodoc.com/presentation_image_h2/990b305e8c6e508ddbe79da756c2f9d4/image-26.jpg)



- Slides: 32

Chapter 4 Atomic Structure Electronic Structure of Atoms

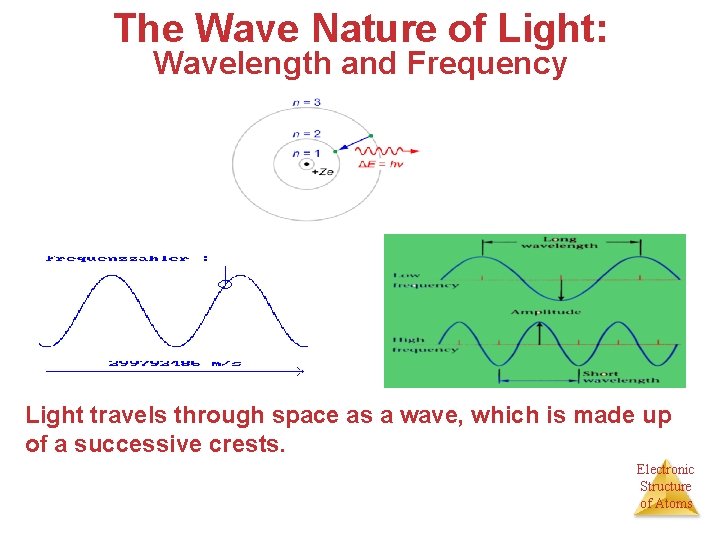
The Wave Nature of Light: Wavelength and Frequency Light travels through space as a wave, which is made up of a successive crests. Electronic Structure of Atoms

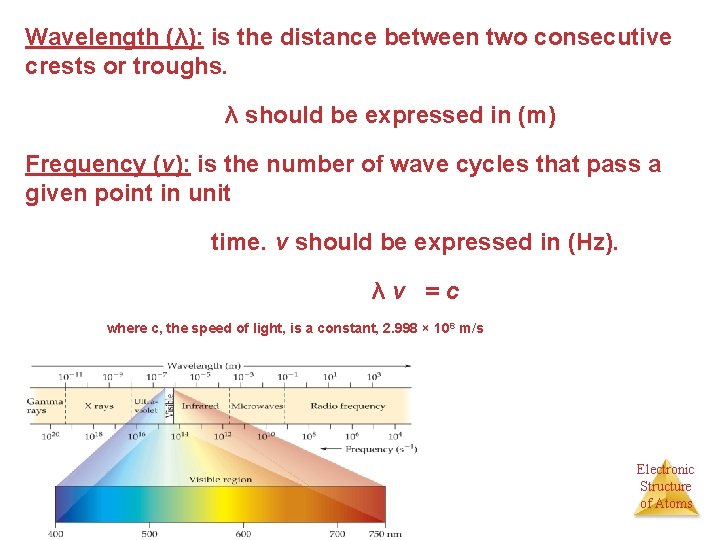
Wavelength (λ): is the distance between two consecutive crests or troughs. λ should be expressed in (m) Frequency (ν): is the number of wave cycles that pass a given point in unit time. ν should be expressed in (Hz). λν =c where c, the speed of light, is a constant, 2. 998 × 10 8 m/s Electronic Structure of Atoms

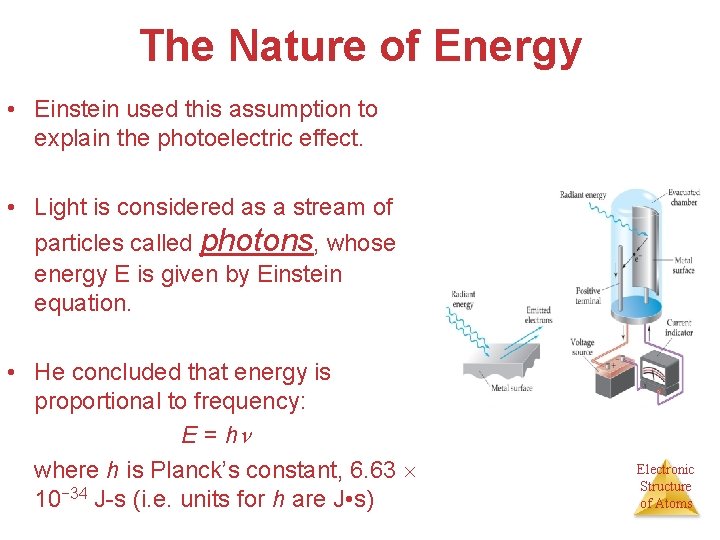
The Nature of Energy • Einstein used this assumption to explain the photoelectric effect. • Light is considered as a stream of particles called photons, whose energy E is given by Einstein equation. • He concluded that energy is proportional to frequency: E = h where h is Planck’s constant, 6. 63 10− 34 J-s (i. e. units for h are J • s) Electronic Structure of Atoms

The Nature of Energy • Therefore, if one knows the wavelength of light, one can calculate the energy in one photon, or packet, of that light: c = E = h Electronic Structure of Atoms

Example • The green light associated with the aurora is emitted by excited oxygen atom at 557. 7 × 10_9 m. What is the frequency of this light and calculate the energy of a photon emitted by an excited oxygen atom. ν =c/λ = 2. 998 × 108 m/s 557. 7 × 10 _9 m = 5. 376 × 10 14 /s E = hc / λ (6. 626 × 10 -34 J. s) (2. 998 × 108 m / s) = = 3. 652 × 10 -19 557. 7 10 -9 m Electronic Structure of Atoms

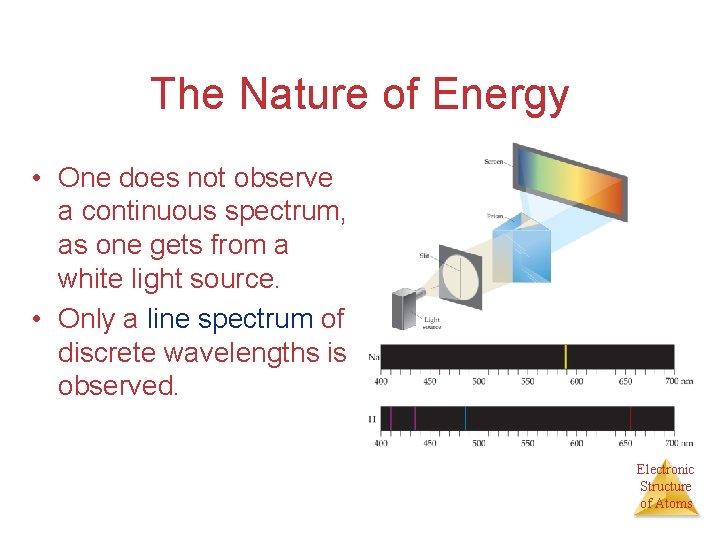
The Nature of Energy • One does not observe a continuous spectrum, as one gets from a white light source. • Only a line spectrum of discrete wavelengths is observed. Electronic Structure of Atoms

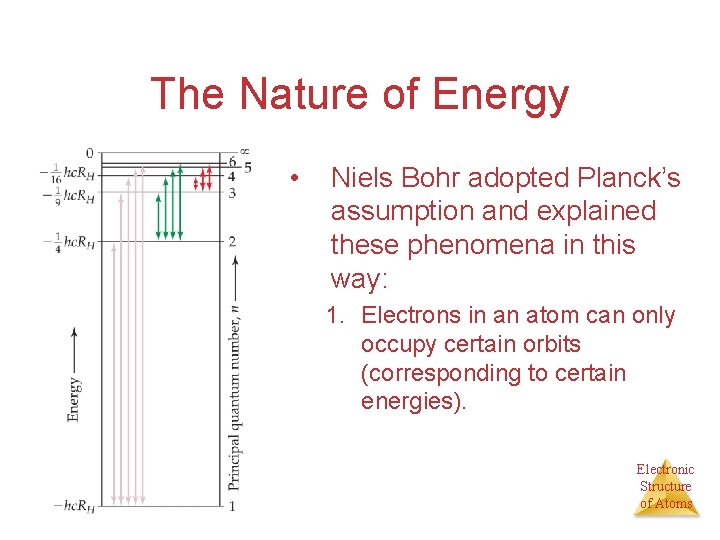
The Nature of Energy • Niels Bohr adopted Planck’s assumption and explained these phenomena in this way: 1. Electrons in an atom can only occupy certain orbits (corresponding to certain energies). Electronic Structure of Atoms

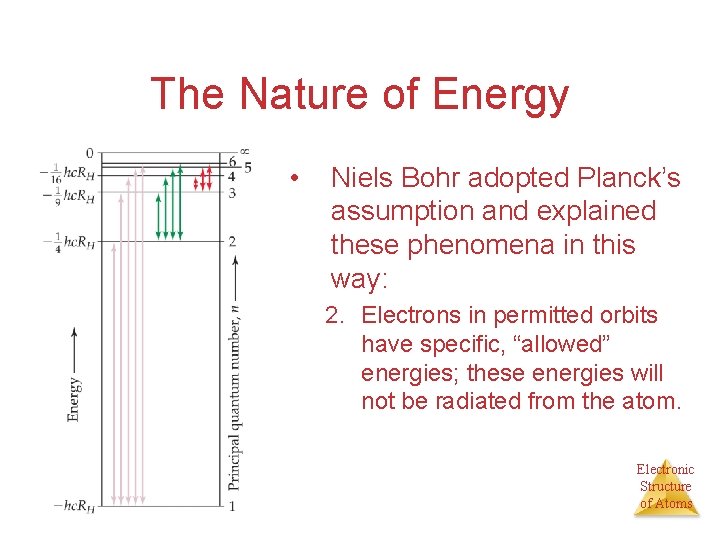
The Nature of Energy • Niels Bohr adopted Planck’s assumption and explained these phenomena in this way: 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. Electronic Structure of Atoms

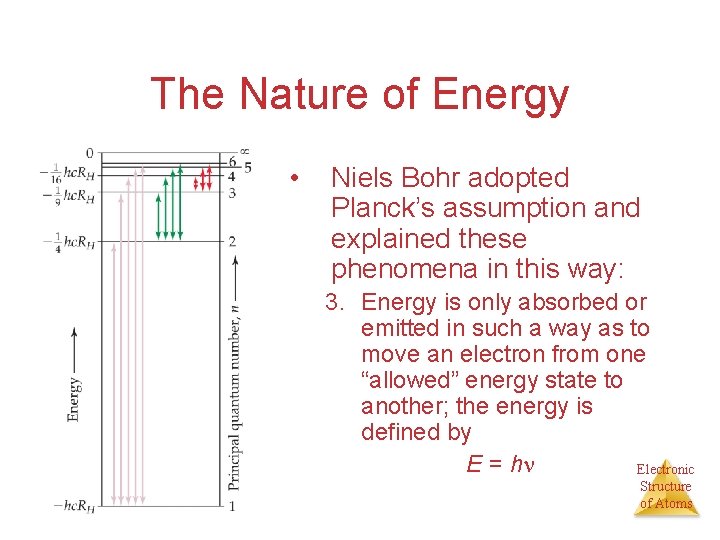
The Nature of Energy • Niels Bohr adopted Planck’s assumption and explained these phenomena in this way: 3. Energy is only absorbed or emitted in such a way as to move an electron from one “allowed” energy state to another; the energy is defined by E = h Electronic Structure of Atoms

The Wave Nature of Matter • Louis de Broglie posited that if light can have material properties, matter should exhibit wave properties. • He demonstrated that the relationship between mass and wavelength was h = mv Electronic Structure of Atoms

Quantum Mechanics • Erwin Schrödinger developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated. • It is known as quantum mechanics. Electronic Structure of Atoms

The Quantum Mechanical Model • Energy is quantized - It comes in chunks. • A quantum is the amount of energy needed to move from one energy level to another. • Since the energy of an atom is never “in between” there must be a quantum leap in energy. Electronic Structure of Atoms

Quantum Numbers • Solving the wave equation gives a set of wave functions, or orbitals, and their corresponding energies. • Each orbital describes a spatial distribution of electron density. • An orbital is described by a set of three quantum numbers. Electronic Structure of Atoms

Principal Quantum Number, n • The principal quantum number, n, describes the energy level on which the orbital resides. • The values of n are integers ≥ 0. Electronic Structure of Atoms

Azimuthal Quantum Number, l • This quantum number defines the shape of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of orbitals. Electronic Structure of Atoms

Azimuthal Quantum Number, l ℓ = (n – 1) Value of l 0 1 2 3 Type of orbital s p d f Electronic Structure of Atoms

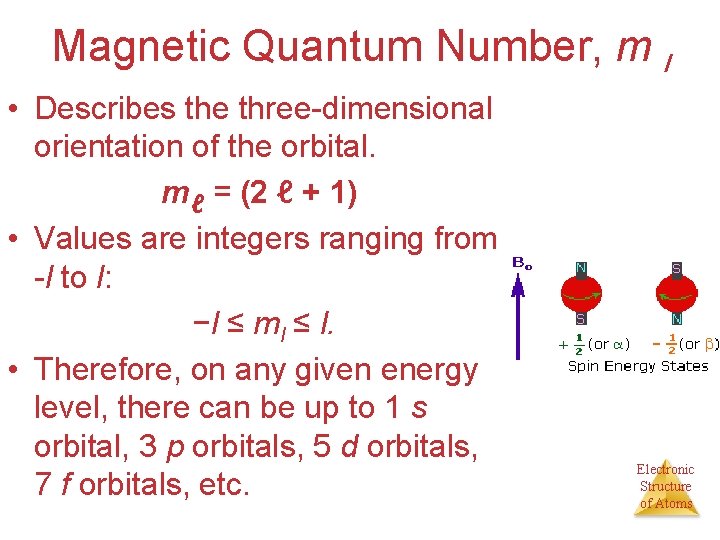
Magnetic Quantum Number, m l • Describes the three-dimensional orientation of the orbital. mℓ = (2 ℓ + 1) • Values are integers ranging from -l to l: −l ≤ ml ≤ l. • Therefore, on any given energy level, there can be up to 1 s orbital, 3 p orbitals, 5 d orbitals, 7 f orbitals, etc. Electronic Structure of Atoms

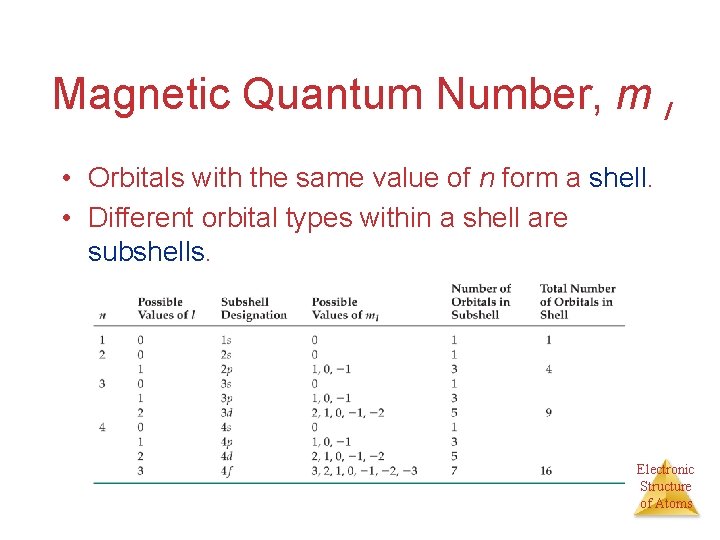
Magnetic Quantum Number, m l • Orbitals with the same value of n form a shell. • Different orbital types within a shell are subshells. Electronic Structure of Atoms

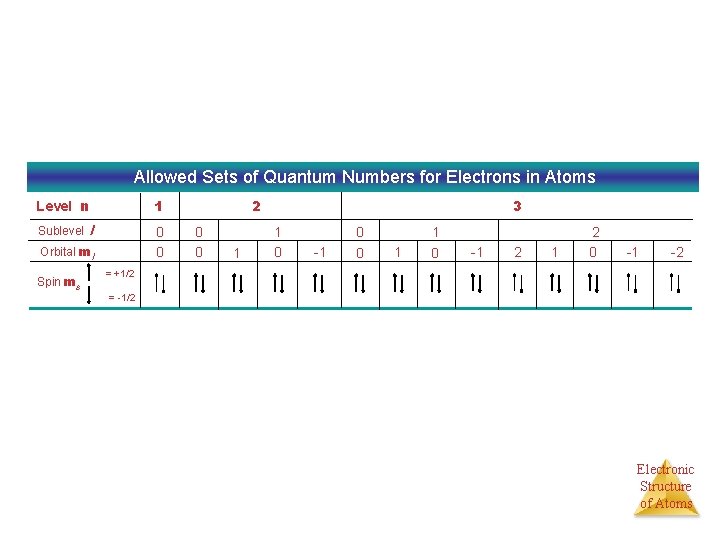
Allowed Sets of Quantum Numbers for Electrons in Atoms Level n Sublevel 1 l 0 0 Orbital ml Spin ms 2 0 0 1 3 1 0 -1 0 0 1 1 0 -1 2 0 -1 -2 = +1/2 = -1/2 Electronic Structure of Atoms

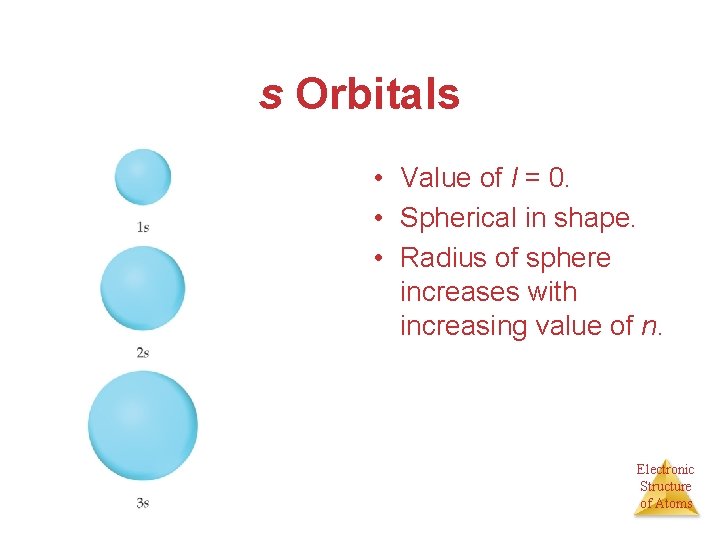
s Orbitals • Value of l = 0. • Spherical in shape. • Radius of sphere increases with increasing value of n. Electronic Structure of Atoms

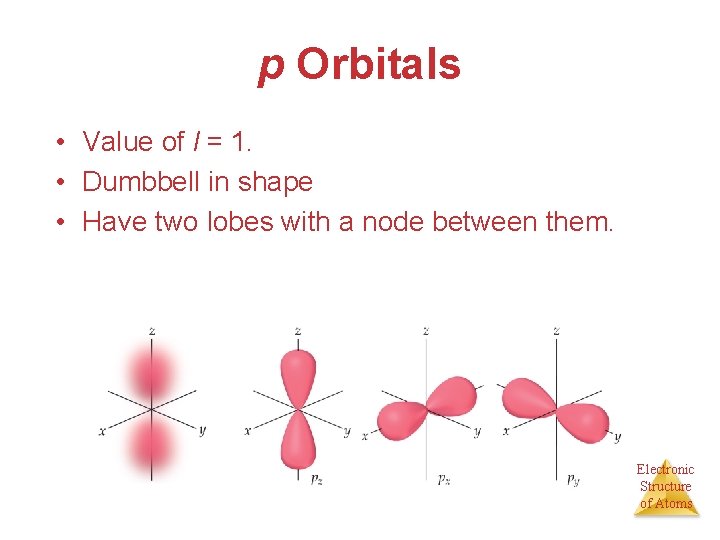
p Orbitals • Value of l = 1. • Dumbbell in shape • Have two lobes with a node between them. Electronic Structure of Atoms

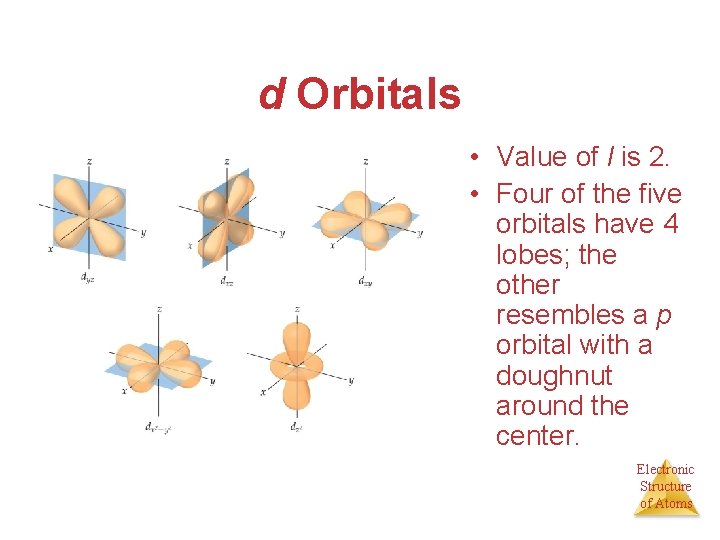
d Orbitals • Value of l is 2. • Four of the five orbitals have 4 lobes; the other resembles a p orbital with a doughnut around the center. Electronic Structure of Atoms

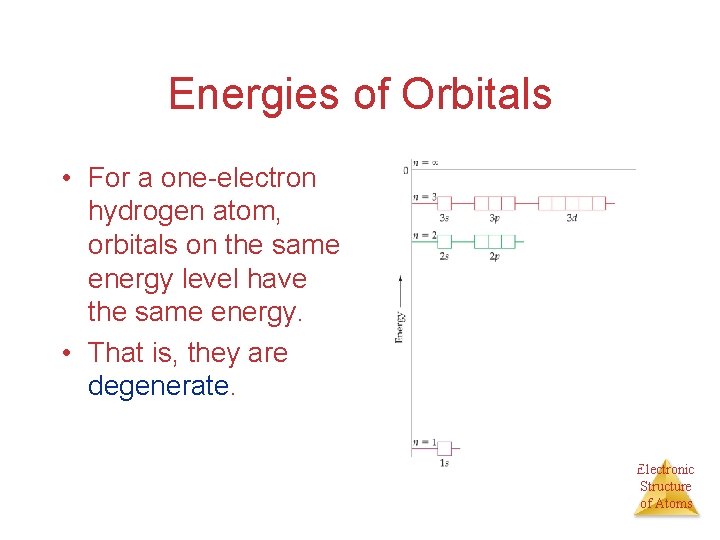
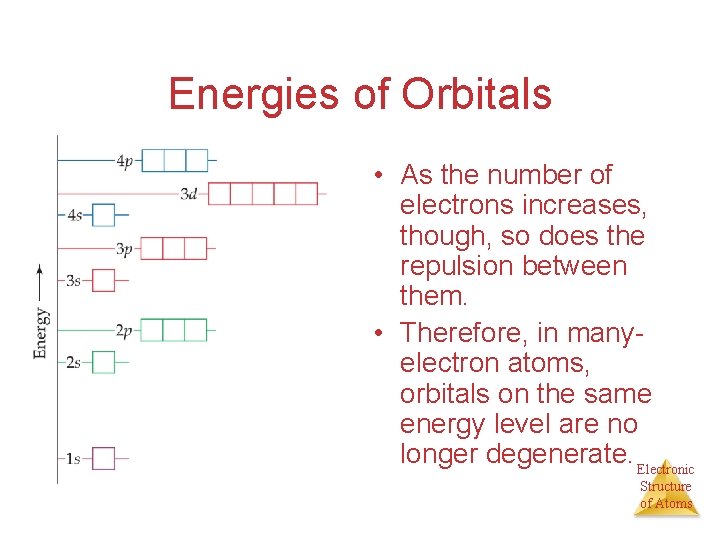
Energies of Orbitals • For a one-electron hydrogen atom, orbitals on the same energy level have the same energy. • That is, they are degenerate. Electronic Structure of Atoms

Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms
![Electron Configuration ØNa 1 s 2 2 p 6 3 s 1 ØNa Ne Electron Configuration ØNa: 1 s 2 2 p 6 3 s 1 ØNa: [Ne]](https://slidetodoc.com/presentation_image_h2/990b305e8c6e508ddbe79da756c2f9d4/image-26.jpg)
Electron Configuration ØNa: 1 s 2 2 p 6 3 s 1 ØNa: [Ne] 3 s 1 Electronic Structure of Atoms

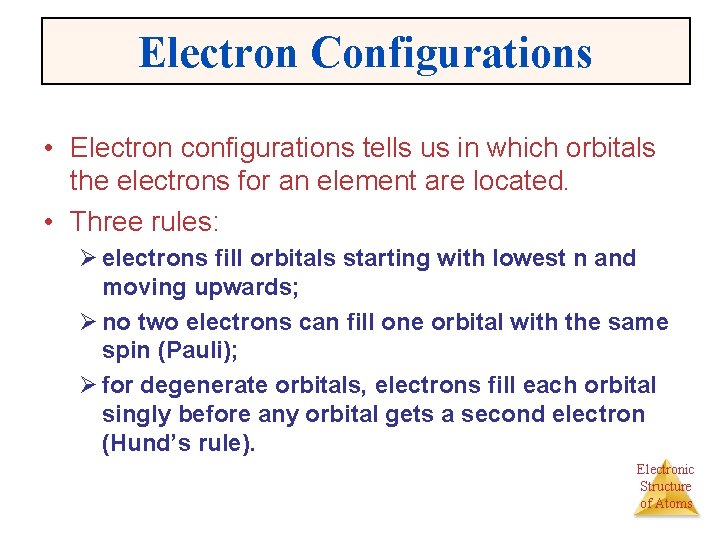
Electron Configurations • Electron configurations tells us in which orbitals the electrons for an element are located. • Three rules: Ø electrons fill orbitals starting with lowest n and moving upwards; Ø no two electrons can fill one orbital with the same spin (Pauli); Ø for degenerate orbitals, electrons fill each orbital singly before any orbital gets a second electron (Hund’s rule). Electronic Structure of Atoms

Writing Electron Configurations • First, determine how many electrons are in the atom. Iron has 26 electrons. • Arrange the energy sublevels according to increasing energy: – 1 s 2 s 2 p 3 s 3 p 4 s 3 d … • Fill each sublevel with electrons until you have used all the electrons in the atom: – Fe: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 • The sum of the superscripts equals the atomic Electronic number of iron (26) Structure of Atoms

Electron Configurations and the Periodic Table • The periodic table can be used as a guide for electron configurations. • The period number is the value of n. • Groups 1 A and 2 A have the s-orbital filled. • Groups 3 A - 8 A have the p-orbital filled. • Groups 3 B - 2 B have the d-orbital filled. • The lanthanides and actinides have the f-orbital filled. Electronic Structure of Atoms

Hund’s Rule “For degenerate orbitals, the lowest energy is attained when the number of electrons with the same spin is maximized. ” Electronic Structure of Atoms

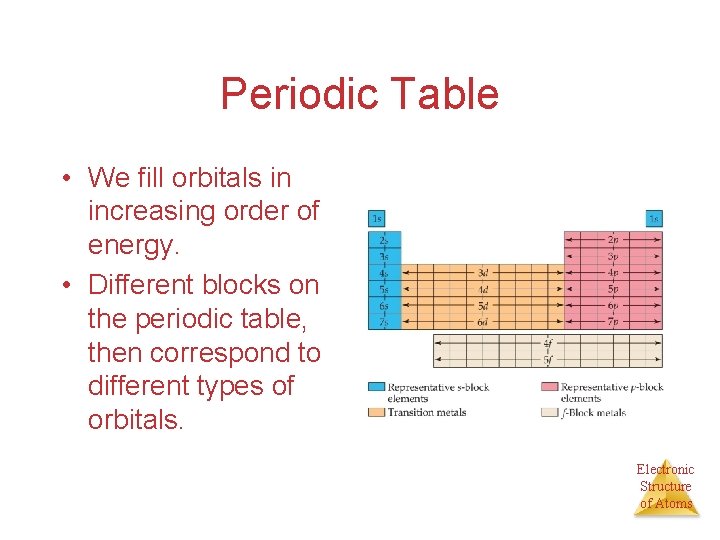
Periodic Table • We fill orbitals in increasing order of energy. • Different blocks on the periodic table, then correspond to different types of orbitals. Electronic Structure of Atoms

ELECTRON SPIN • 1920 --chemists realized that since electrons interact with a magnetic field, there must be one more concept to explain the behavior of electrons in atoms. • ms--the 4 th quantum number; accounts for the reaction of electrons in a magnetic field MAGNETISM • magnetite--Fe 3 O 4, natural magnetic oxide of iron • 1600 --William Gilbert concluded the earth is also a large spherical magnet with magnetic south at the north pole (Santa's habitat). • NEVER FORGET: opposites attract & likes repel PARAMAGNETISM AND UNPAIRED ELECTRONS • diamagnetic--not magnetic [magnetism dies]; in fact they are slightly repelled. All electrons are PAIRED. • paramagnetic--attracted to a magnetic field; lose their magnetism when removed from the magnetic field; HAS ONE OR MORE UNPAIRED ELECTRONS Electronic Structure of Atoms