Atoms Atomic Structure Chapter 4 Atomic History Democritus

















- Slides: 37

Atoms & Atomic Structure Chapter 4

Atomic History: Democritus • Democritus – – Greece; 460 -370 B. C. Thought atoms were indivisible Thought matter was composed of tiny indivisible particles called atoms First to suggest atoms existed

Atomic History: Dalton • John Dalton – England 1766 -1844 – Performed experiments to develop Atomic Theory that validated Democritus’ ideas

Dalton’s Atomic Theory 1. All elements composed of atoms 2. Atoms of the same element are identical, atoms of different elements are different 3. Atoms of different elements can be physically mixed (mixtures) or chemically combined (compounds) to form whole number ratios 4. Chemical reactions occur when atoms are separated, joined or rearranged by breaking and remaking bonds

Atoms • Atom: smallest particle of an element that retains the properties of that element

Atomic Size • Atoms are very small – 1 copper penny contains 2. 4 x 1022 atoms of copper – Atomic Radii are between 5 x 10 -2 nm & 2 x 10 -1 nm – Observable using scanning tunneling microscopes – 10 Hydrogen atoms in a line measure 1 nanometer across

Subatomic Particles • Atoms can be broken down into even smaller more fundamental particles called subatomic particles Three subatomic particles make-up atom • – – – Protons (+) Neutrons (0) Electrons (-)

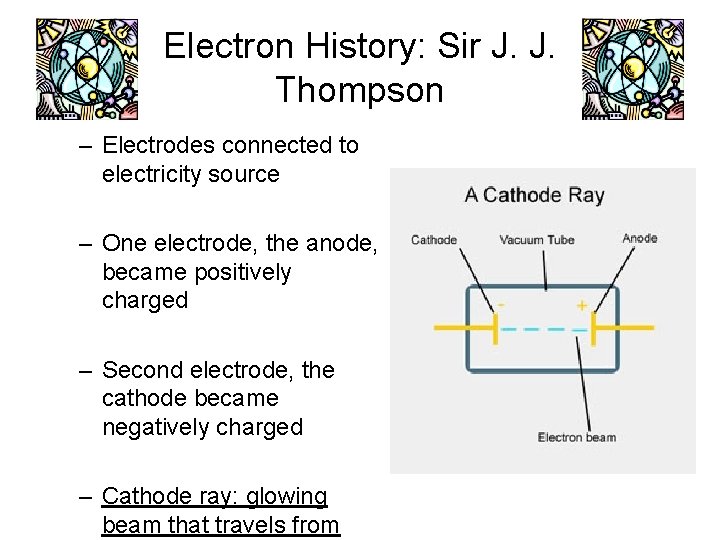
Electron History: Sir J. J. Thomson • • England 1856 -1940 Discovered electrons in 1897 – – Performed experiment involving the flow of electric current through gases Sealed gas in glass tubes with electrodes at each end.

Electron History: Sir J. J. Thompson – Electrodes connected to electricity source – One electrode, the anode, became positively charged – Second electrode, the cathode became negatively charged – Cathode ray: glowing beam that travels from

Electron • Electrons: negatively charge subatomic particle found in the electron cloud of the atom – Charge: 1 unit of negative charge – Mass: 9. 11 x 10 -28 g; 1/1840 the mass of a proton or a hydrogen atom – Atomic Mass: 0. 00055 amu (Atomic Mass Unit)

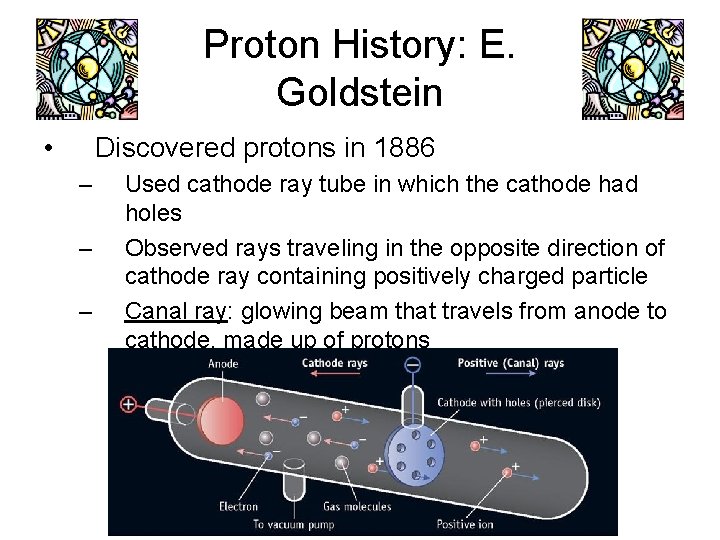
Proton History: E. Goldstein • Discovered protons in 1886 – – – Used cathode ray tube in which the cathode had holes Observed rays traveling in the opposite direction of cathode ray containing positively charged particle Canal ray: glowing beam that travels from anode to cathode, made up of protons

Protons • Protons: positively charged subatomic particle found in the nucleus of the atom – Charge: 1 unit of positive charge – Mass: 1. 67 x 10 -24 g; – Atomic Mass: 1. 0 amu (Atomic Mass Unit)

Neutron History • Sir James Chadwick (1891 -1974) – English Physicist – Discovered neutron in 1932 – Accounted for discrepancies in atomic mass measurements of previous experiments

Neutrons • Neutrons: neutral subatomic particle found in the nucleus of an atom – Charge: no charge – Mass: 1. 67 x 10 -24 g; – Atomic Mass: 1. 0 amu (Atomic Mass Unit)

Rutherford

Ernest Rutherford (18711937) • Experiment – – – • Directed a beam of alpha particles at a thin sheet of gold foil Alpha particles: helium atoms that have lost two electrons and have a double positive charge from the remaining two protons Particles moved in one of three ways Small fraction of alpha particles bounce off foil (repelled) Small fraction of alpha particles attracted to foil (almost never) Majority of particles pass through foil Repulsive movement suggested positively

Robert A Millikan • 1863 -1953 • Experiment: motion of a charged drop of oil in an electric field • Found the quantity of charge carried by electrons • Calculated the mass of an electron

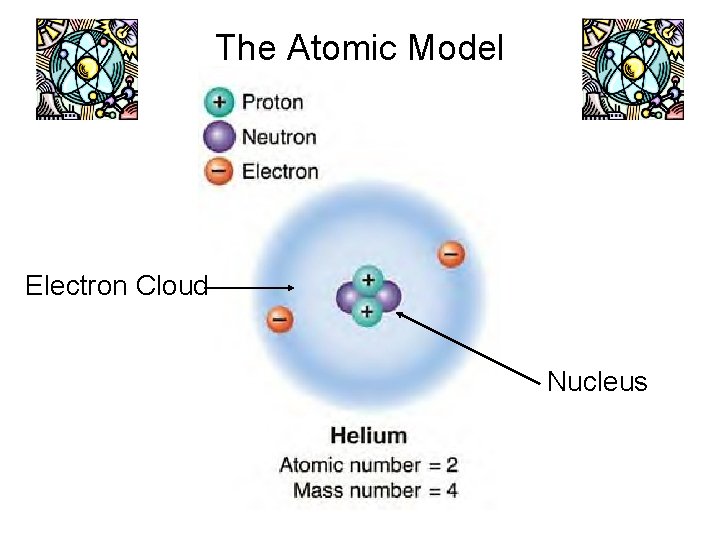
Atomic Model

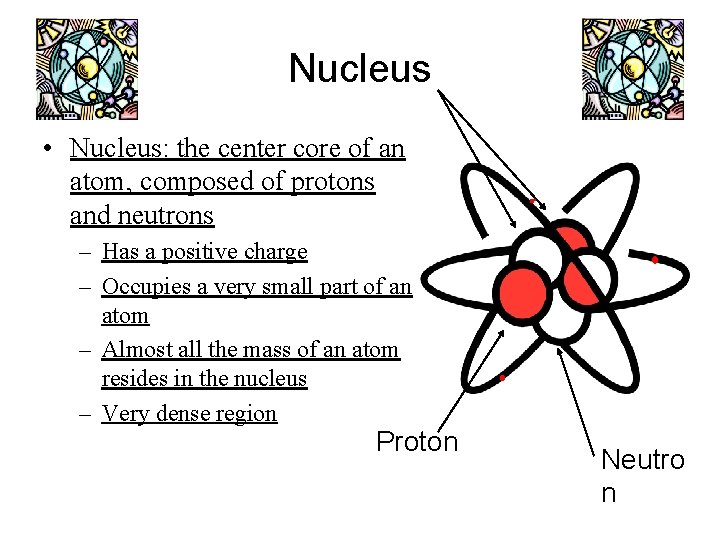
Nucleus • Nucleus: the center core of an atom, composed of protons and neutrons – Has a positive charge – Occupies a very small part of an atom – Almost all the mass of an atom resides in the nucleus – Very dense region Proton Neutro n

Electron Cloud • Electron cloud: region surrounding nucleus that contains electron. • Most of an atom is empty space.

The Atomic Model Electron Cloud Nucleus

Atomic Number • Atomic Number: the number of protons in the nucleus of an atom of an element – No 2 elements have the same atomic number – Atomic Number identifies an element – Number of protons in an atom = Number of electrons in an atom – Atomic number values increase left to right and top to bottom in the periodic table

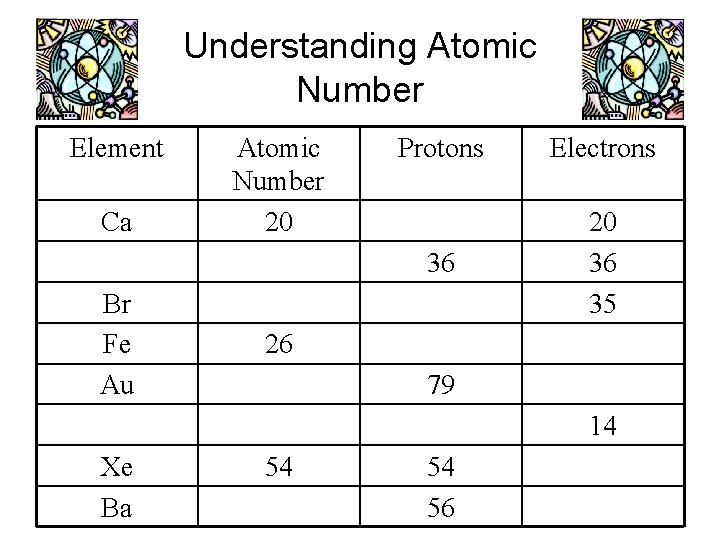
Understanding Atomic Number Element Ca Br Fe Au Atomic Number 20 Protons Electrons 36 20 36 35 26 79 14 Xe Ba 54 54 56

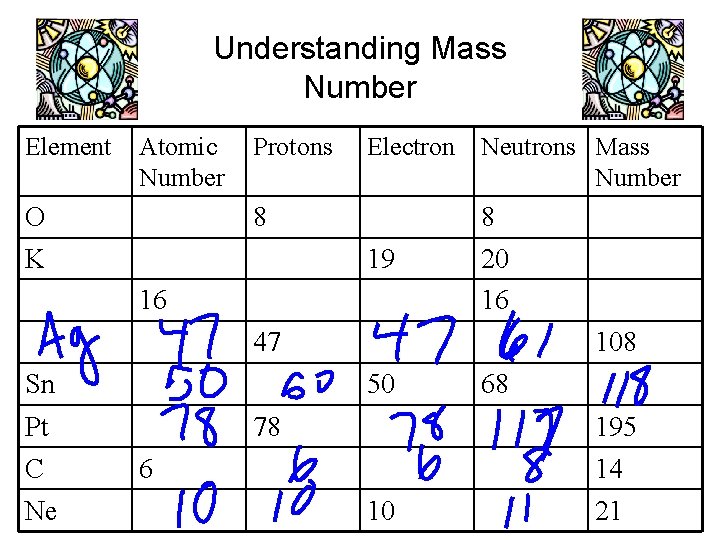
Mass Number • Mass Number: the total number of protons and neutrons in the nucleus of an atom • Mass Number – Atomic Number = the Number of Neutrons

Understanding Mass Number Element Atomic Number O Protons Electron 8 K Neutrons Mass Number 8 19 16 20 16 47 Sn 50 Pt C Ne 108 78 68 195 6 14 10 21

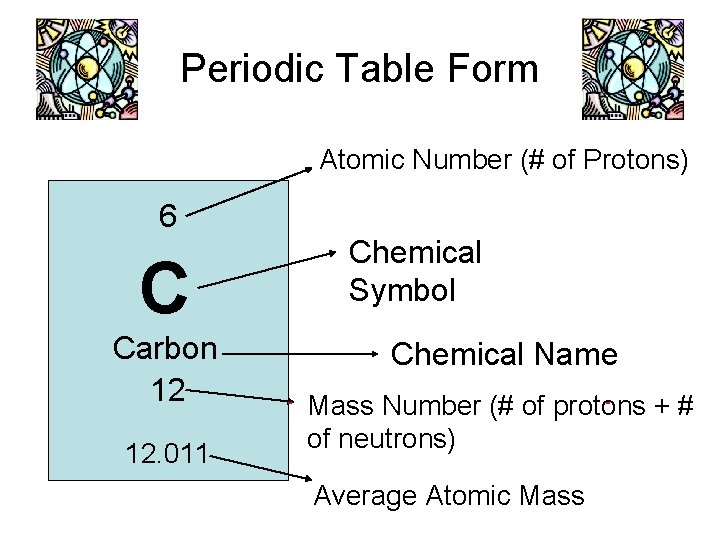
Periodic Table Form Atomic Number (# of Protons) 6 C Carbon 12 12. 011 Chemical Symbol Chemical Name Mass Number (# of protons + # of neutrons) Average Atomic Mass

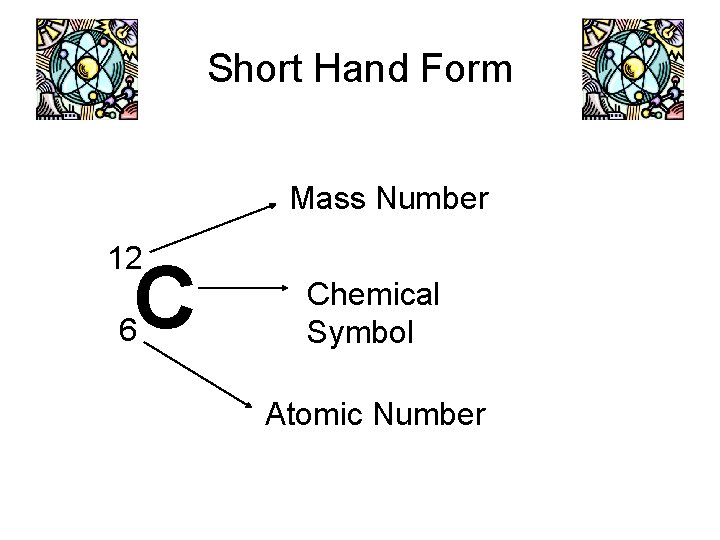
Short Hand Form Mass Number 12 C 6 Chemical Symbol Atomic Number

Isotopes • Isotope: atoms that have the same number of protons but different numbers of neutrons in the nucleus. – Isotopes of the same element are chemically the same because they have the same numbers of protons and electrons – Mass Numbers of isotopes of the same element will be different

Isotopes • Each element can Have more than 1 isotope • In nature elements occur in mixtures of 2 or more isotopes. – Hydrogen has 3 isotopes • Hydrogen-1 (1 H) called hydrogen • Hydrogen-2 (2 H) called deuterium • Hydrogen-3 (3 H) called tritium – Carbon has several isotopes • Carbon-12 (12 C) • Carbon-13 (13 C) • Carbon-14 (14 C)

Natural Percent Abundance • Natural percent abundance: is the measurement of the natural occurrence of each isotope written as a percent

Atomic Mass • Atomic mass: a weighted average mass of the atoms in a naturally occurring sample of the element – Reflects both the mass and the relative natural percent abundance of the isotope – Atomic Mass Unit (amu): one twelfth the mass of a carbon atom or the mass of 1 proton – In nature each isotope has a fixed mass and a natural percent abundance

Calculating Atomic Mass • To calculate atomic mass the following things are required: – Number of stable isotopes of that element – Mass of each isotope – Natural percent abundance of each isotope

Calculating Average Atomic Mass • Step 1 – List the natural isotopes in short hand form with their mass in amu and natural percent abundances • Step 2 – Multiply the mass of each isotope by the corresponding natural percent abundance in decimal form. • Step 3 – Add the products from step 2 and that is your average atomic mass

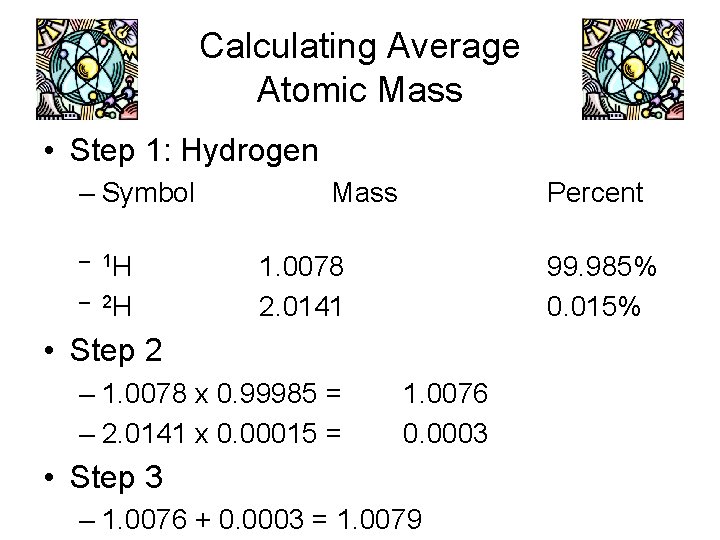
Calculating Average Atomic Mass • Step 1: Hydrogen – Symbol – 1 H – 2 H Mass Percent 1. 0078 2. 0141 99. 985% 0. 015% • Step 2 – 1. 0078 x 0. 99985 = – 2. 0141 x 0. 00015 = 1. 0076 0. 0003 • Step 3 – 1. 0076 + 0. 0003 = 1. 0079

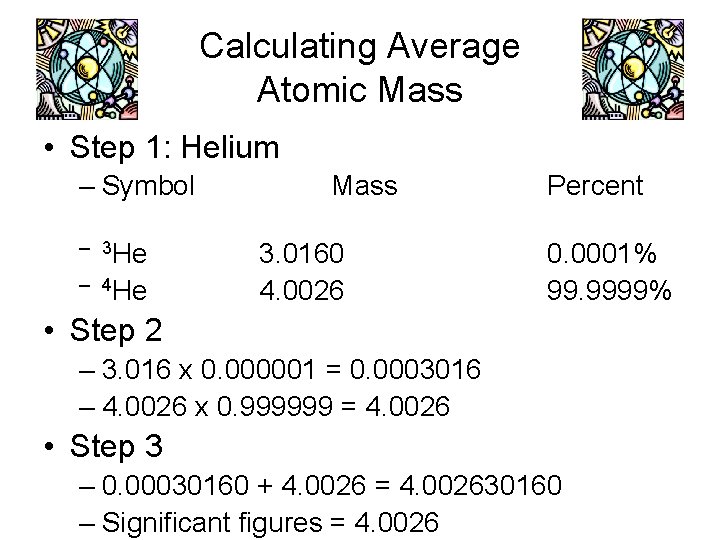
Calculating Average Atomic Mass • Step 1: Helium – Symbol – 3 He – 4 He Mass 3. 0160 4. 0026 Percent 0. 0001% 99. 9999% • Step 2 – 3. 016 x 0. 000001 = 0. 0003016 – 4. 0026 x 0. 999999 = 4. 0026 • Step 3 – 0. 00030160 + 4. 0026 = 4. 002630160 – Significant figures = 4. 0026

Calculating Average Atomic Mass • Calculate the Average Atomic Mass for the following elements: – Carbon – Nitrogen – Oxygen – Sulfur – Chlorine – Zinc – Neon

Periodic Preview • Periodic Table: arrangement of elements in which elements are separated into groups based on repeating properties • Group/Family: vertical column of periodic table, same number of valance electrons • Period: horizontal row of periodic table