The Atomic Structure of Atoms Democritus Hypothesizing the






- Slides: 15

The Atomic Structure of Atoms

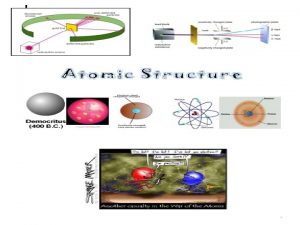
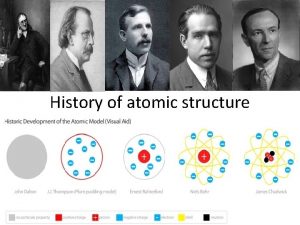
Democritus: Hypothesizing the Atom u u u Around 460 B. C. , Democritus first developed the idea of atoms as the smallest possible bit of matter. The word “atom” came from the Greek word atomos meaning indivisible Greek philosophers laid the ground work for the modern atomic theory 2

Dalton: The Atomic Theory u u John Dalton, in the early 1800’s, developed the “atomic theory” from observations gathered from many experiments. Fun Fact: Dalton was a school teacher and chemist! 3

Dalton’s Atomic Theory: u 1. All elements are composed of atoms. Atoms cannot be divided or destroyed. – (Now we know atoms can be divided) u 2. Atoms of the same element are exactly alike. Atoms of different elements are different. – (Now we know that atoms of the same element that are not exactly alike are called isotopes – they have a diff. # of neutrons) u 3. The atoms of two or more elements can join together to form compounds.

J. J. Thomson: 1856 -1940 u Discovered the electron. u He was awarded with the Nobel Prize in Physics in 1906. 5

Lord Ernest Rutherford: 1871 -1937 u Ernest Rutherford was once a student of JJ Thomson u Discovered that most of the atom is made up of "empty space” where the electrons move u He discovered and named both the nucleus and protons 6

James Chadwick: 1891 -1974 u James Chadwick was a student of Rutherford u Discovered the neutron u He found neutrons to have essentially the same mass as protons u He was awarded the Nobel Prize in physics in 1935 7

Niels Bohr: early 1900’s u Discovered electrons traveled in energy levels around the nucleus. u The lowest energy level is closest to the nucleus and holds up to 2 electrons. u Higher energy levels are father from the nucleus and can contain more electrons 8

REVIEW: Early Atomic Chemists ü Democritus ü ü Dalton ü ü Named the nucleus, proton, and described the “empty space” where electrons travel Chadwick ü ü Discovered electron Rutherford ü ü Atomic Theory Thomson ü ü Named the atom Discovered neutron Bohr ü Discovered electron energy levels 9

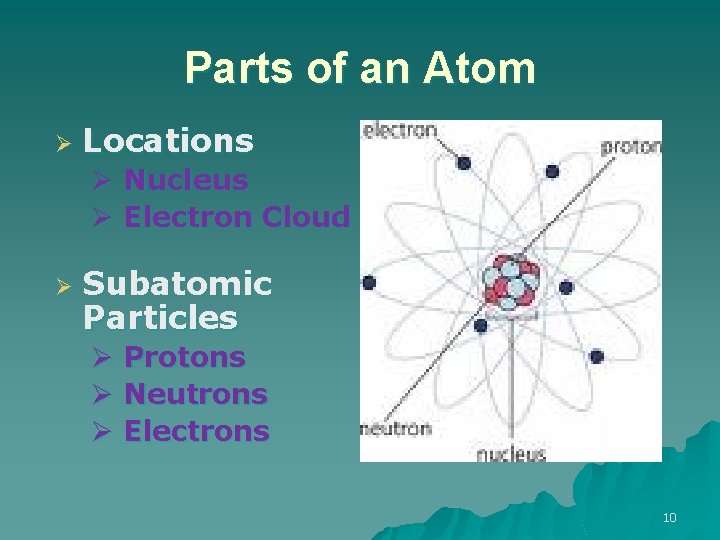
Parts of an Atom Ø Locations Ø Nucleus Ø Electron Cloud Ø Subatomic Particles Ø Protons Ø Neutrons Ø Electrons 10

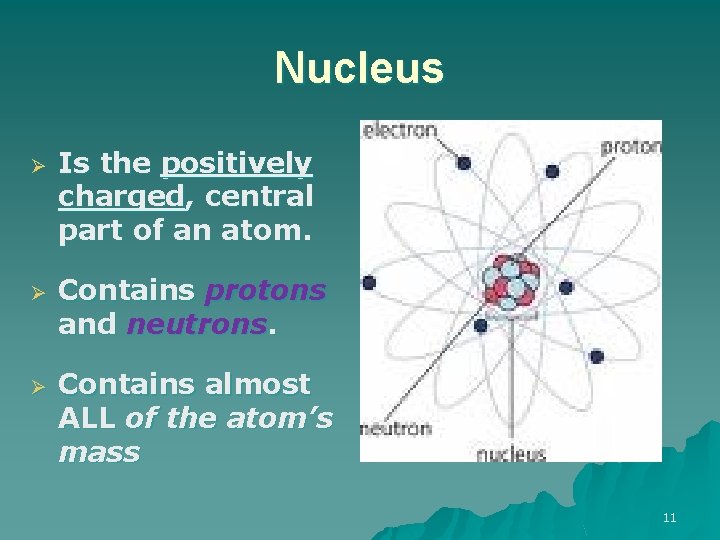
Nucleus Ø Ø Ø Is the positively charged, central part of an atom. Contains protons and neutrons. Contains almost ALL of the atom’s mass 11

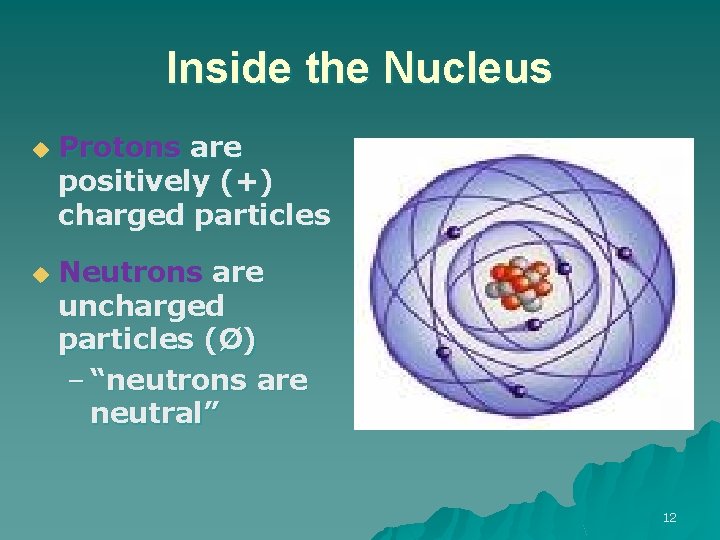
Inside the Nucleus u u Protons are positively (+) charged particles Neutrons are uncharged particles (Ø) – “neutrons are neutral” 12

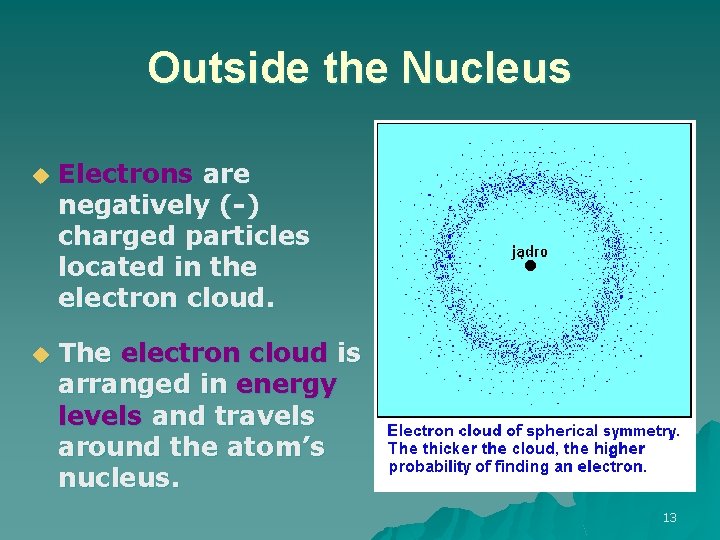
Outside the Nucleus u Electrons are negatively (-) charged particles located in the electron cloud. u The electron cloud is arranged in energy levels and travels around the atom’s nucleus. 13

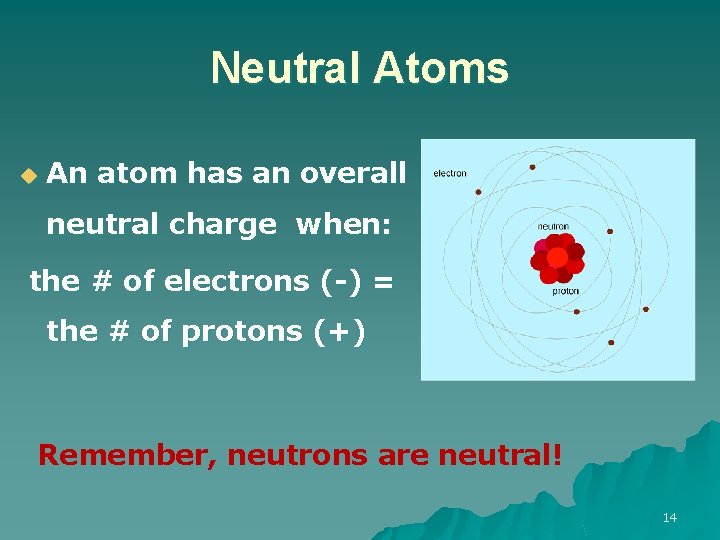
Neutral Atoms u An atom has an overall neutral charge when: the # of electrons (-) = the # of protons (+) Remember, neutrons are neutral! 14

HAVE A WONDERFUL DAY!! 15
 A suggested solution to a problem or question
A suggested solution to a problem or question How did democritus characterize atoms?
How did democritus characterize atoms? Democritus diagram
Democritus diagram Democritus atomic theory
Democritus atomic theory Timeline of democritus
Timeline of democritus Democritus atomic model
Democritus atomic model Democritus atomic model diagram
Democritus atomic model diagram History of the atom scientists
History of the atom scientists Aristotle atomic
Aristotle atomic Periodic table regents
Periodic table regents Matterville answer key
Matterville answer key Reference table periodic table
Reference table periodic table Atoms family song
Atoms family song Http sciencespot net
Http sciencespot net The atoms family atomic math challenge
The atoms family atomic math challenge Matterville answer key
Matterville answer key