Chapter 4 Atomic Structure Atomic Theories Atoms are



![Electron Wave Theory [Quantum Mechanical Model] ◦ Electron Cloud (electrons DO NOT move Electron Wave Theory [Quantum Mechanical Model] ◦ Electron Cloud (electrons DO NOT move](https://slidetodoc.com/presentation_image_h2/11867c157c0c2f39c864faae25e5f0d3/image-14.jpg)
- Slides: 14

Chapter 4 “Atomic Structure”

Atomic Theories Atoms are so small that they cannot be observed directly. Scientists had to use the experimental data available at the time to construct a model of the atom. Therefore, the MODEL of the atom has changed over time.

Ancient Philosophy Who: Aristotle When: More than 2000 years ago Where: Greece What: Aristotle believed in 4 elements – Earth, Air, Fire, and Water.

Defining the Atom The Greek philosopher Democritus (460 B. C. – 370 B. C. ) was among the first to suggest the existence of atoms (from the Greek word “atomos”) ◦ He believed that atoms were indivisible and indestructible ◦ His ideas did agree with later scientific theory, but did not explain chemical behavior, and was not based on the scientific method – but just philosophy

Dalton’s Atomic Theory (experiment based!) 1) All elements are composed of tiny indivisible particles called atoms 2) Atoms of the same element are identical. Atoms of any one John Dalton element are different from those of (1766 – 1844) any other element. 3) Atoms of different elements combine in simple whole-number ratios to form chemical compounds 4) In chemical reactions, atoms are combined, separated, or rearranged – but never changed into atoms of another element.

Not all of Dalton’s claims held up to the scrutiny of experimentation. We now understand that: ◦ atoms are divisible into subatomic particles: Electrons, protons, and neutrons (among many others) ◦ Not every atom of an element has an identical mass Ex: Carbon has several different ‘versions’ with the same # protons, but different # neutrons

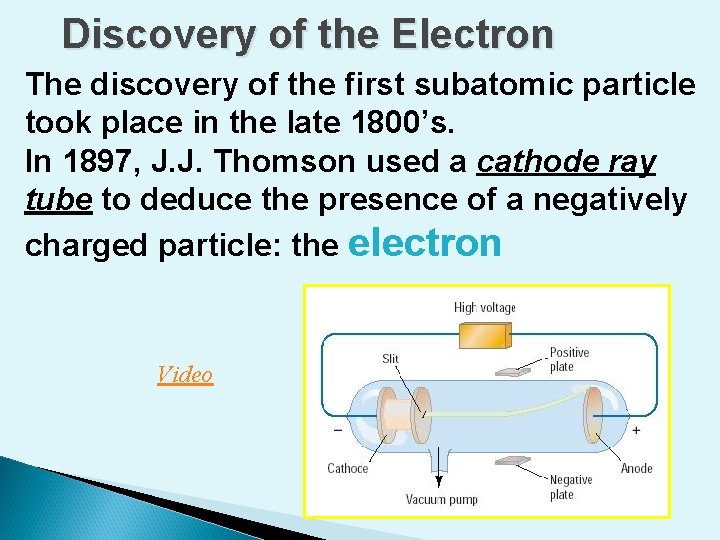
Discovery of the Electron The discovery of the first subatomic particle took place in the late 1800’s. In 1897, J. J. Thomson used a cathode ray tube to deduce the presence of a negatively charged particle: the electron Video

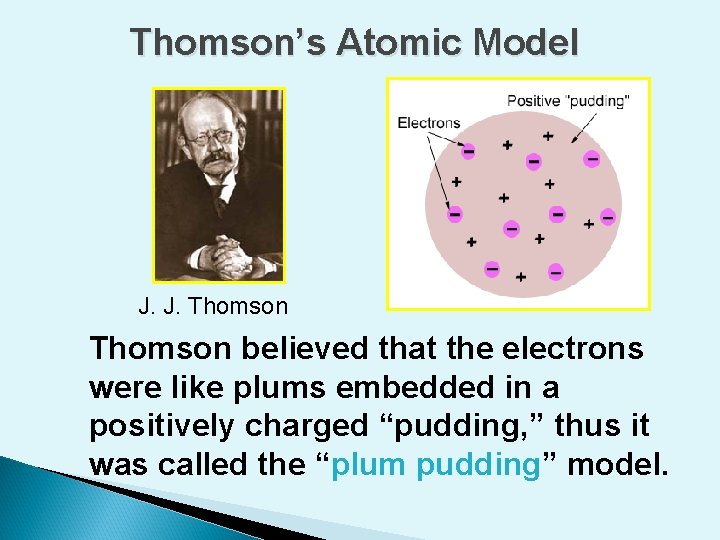
Thomson’s Atomic Model J. J. Thomson believed that the electrons were like plums embedded in a positively charged “pudding, ” thus it was called the “plum pudding” model.

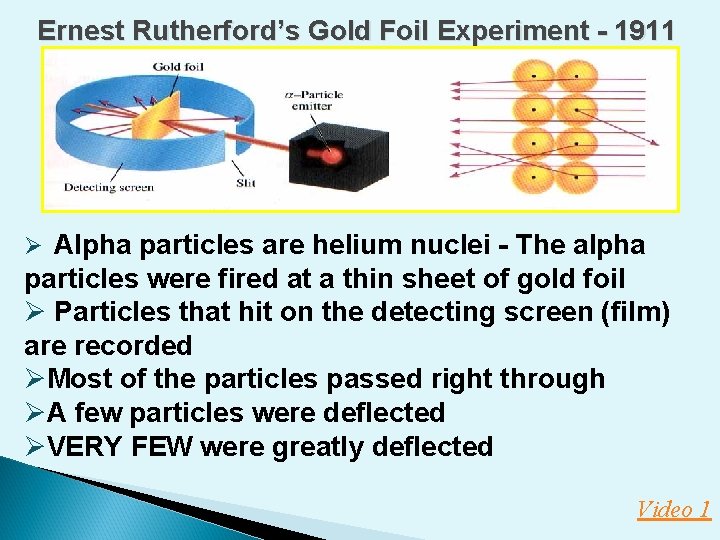
Ernest Rutherford’s Gold Foil Experiment - 1911 Ø Alpha particles are helium nuclei - The alpha particles were fired at a thin sheet of gold foil Ø Particles that hit on the detecting screen (film) are recorded ØMost of the particles passed right through ØA few particles were deflected ØVERY FEW were greatly deflected Video 1

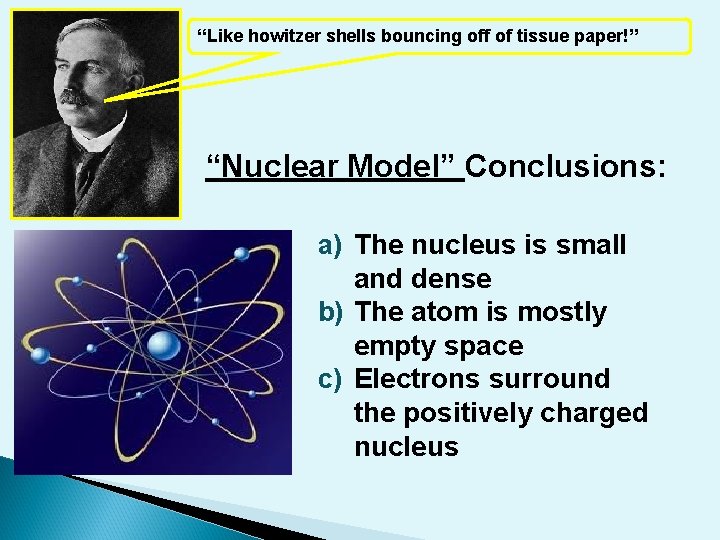
“Like howitzer shells bouncing off of tissue paper!” “Nuclear Model” Conclusions: a) The nucleus is small and dense b) The atom is mostly empty space c) Electrons surround the positively charged nucleus

In 1932, the English physicist James Chadwick discovered yet another subatomic particle: ◦ The neutron is electrically neutral ◦ Its mass is nearly equal to the proton Therefore, the subatomic particles are: ◦ the electron, proton, and neutron.

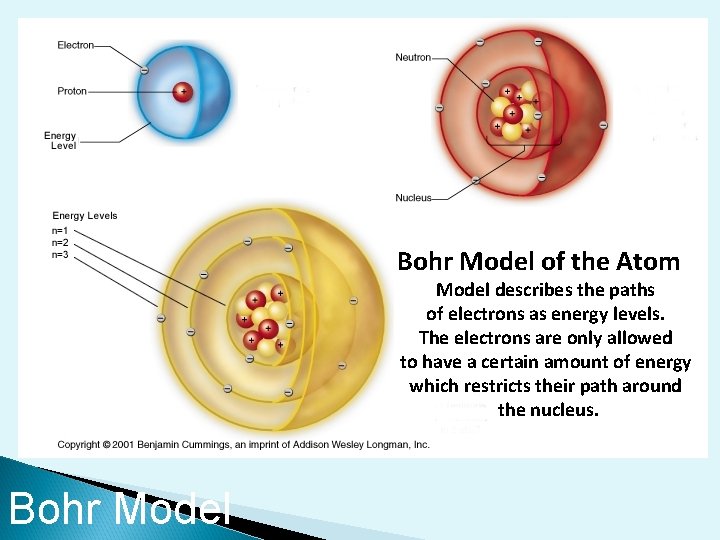
The Bohr Model {orbits} ØElectrons move in a fixed path (certain distances) around the nucleus ØPlanetary model with energy levels {like the rungs of a ladder} Niels Bohr The basis for what orbit an electron is allowed is entirely based on how much energy the electron has If it has any more energy or any less energy it would be forced to be on a different path of different energy

Bohr Model of the Atom Model describes the paths of electrons as energy levels. The electrons are only allowed to have a certain amount of energy which restricts their path around the nucleus. Bohr Model
![Electron Wave Theory Quantum Mechanical Model Electron Cloud electrons DO NOT move Electron Wave Theory [Quantum Mechanical Model] ◦ Electron Cloud (electrons DO NOT move](https://slidetodoc.com/presentation_image_h2/11867c157c0c2f39c864faae25e5f0d3/image-14.jpg)
Electron Wave Theory [Quantum Mechanical Model] ◦ Electron Cloud (electrons DO NOT move in a definite path, but regions, called orbitals) ◦ Scientists cannot predict where they will be at any given moment. Electrons travel so fast, they appear to form a ‘cloud’ around the nucleus.