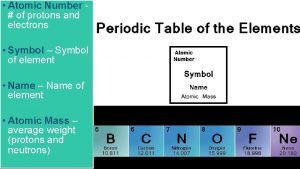
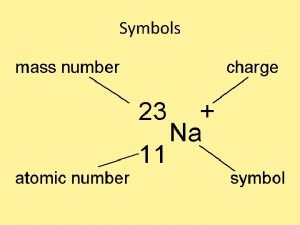
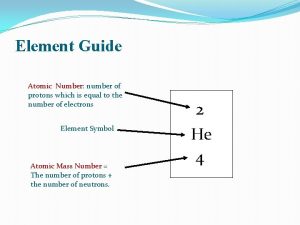
Atomic Number of protons and electrons Symbol Symbol
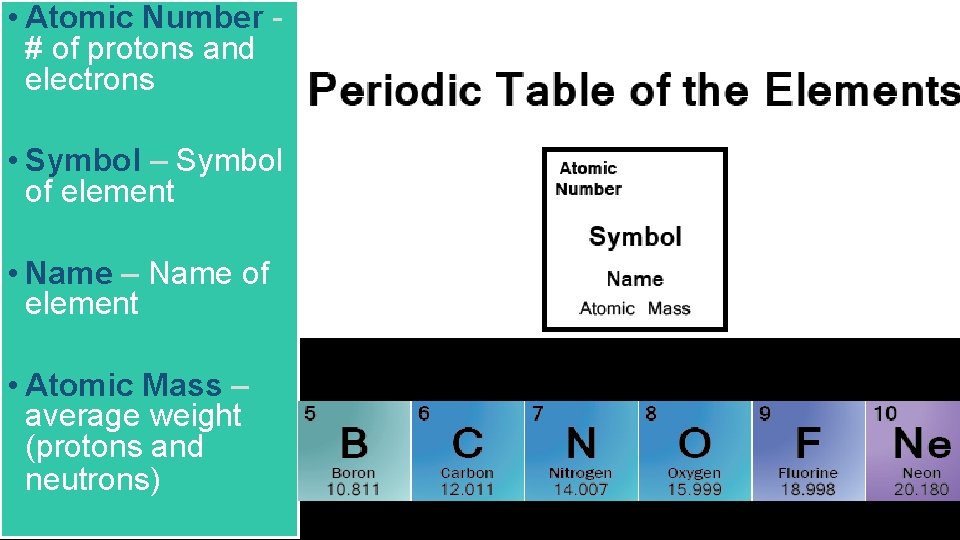
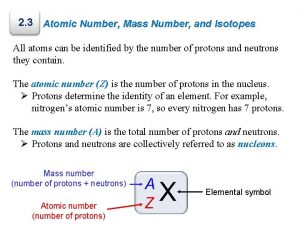
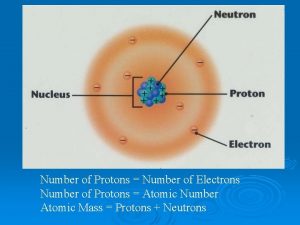
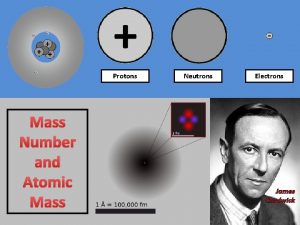
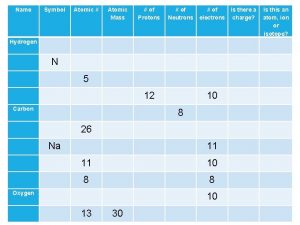
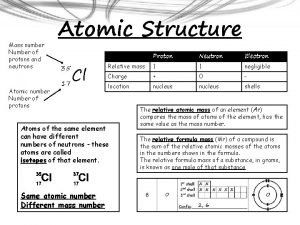
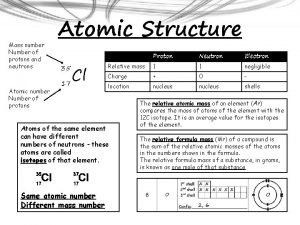
• Atomic Number # of protons and electrons • Symbol – Symbol of element • Name – Name of element • Atomic Mass – average weight (protons and neutrons)
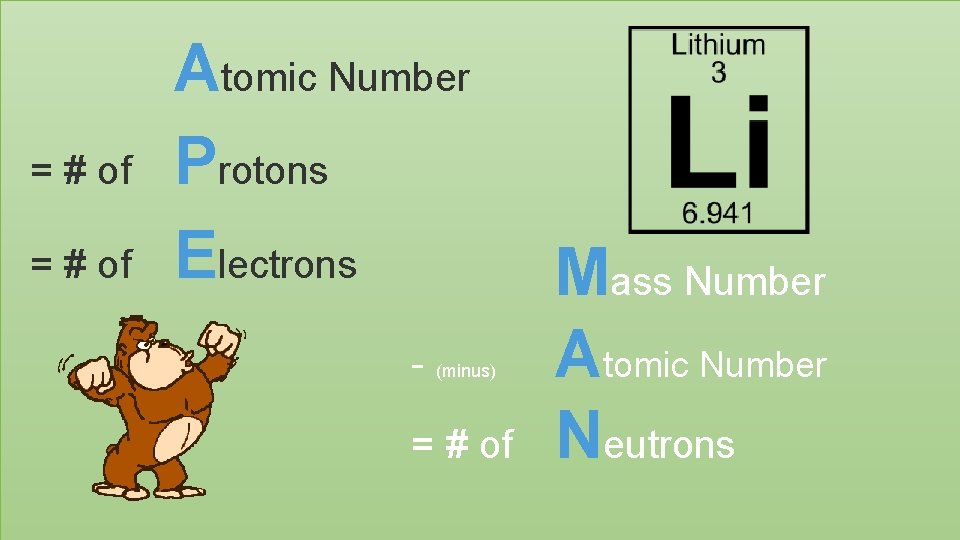
= # of Atomic Number Protons Electrons - (minus) = # of Mass Number Atomic Number Neutrons

WHAT IS AN ATOM? • Atoms are tiny particles too small to see with your eyes or basic microscope • Atoms have mass and take up space, therefore… • Atoms are the basic building blocks of matter – all matter is composed of atoms.
WHAT IS AN ATOM? https: //www. youtube. com/watch? v=o. SCX 78 -8 -q 0
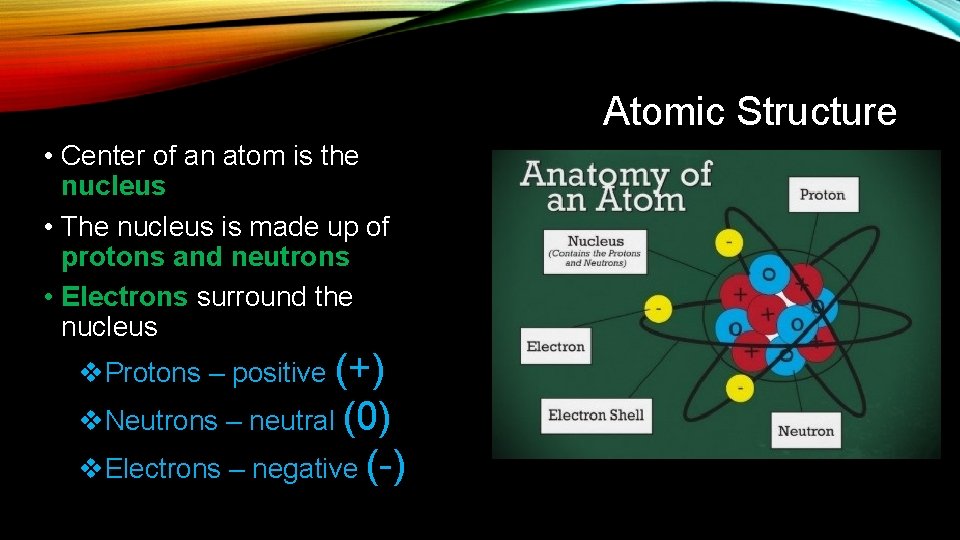
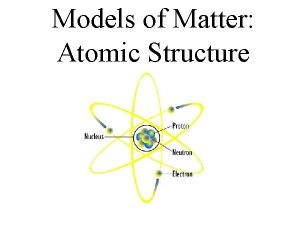
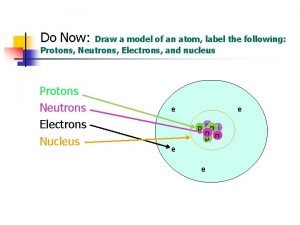
Atomic Structure • Center of an atom is the nucleus • The nucleus is made up of protons and neutrons • Electrons surround the nucleus v. Protons – positive (+) v. Neutrons – neutral (0) v. Electrons – negative (-)

COMPOUNDS HAVE FORMULAS H 2 O = Water H 2 O 2 = Hydrogen peroxide • The subscript represents the number of atoms for that element. NEVER ERASE A SUBSCRIPT
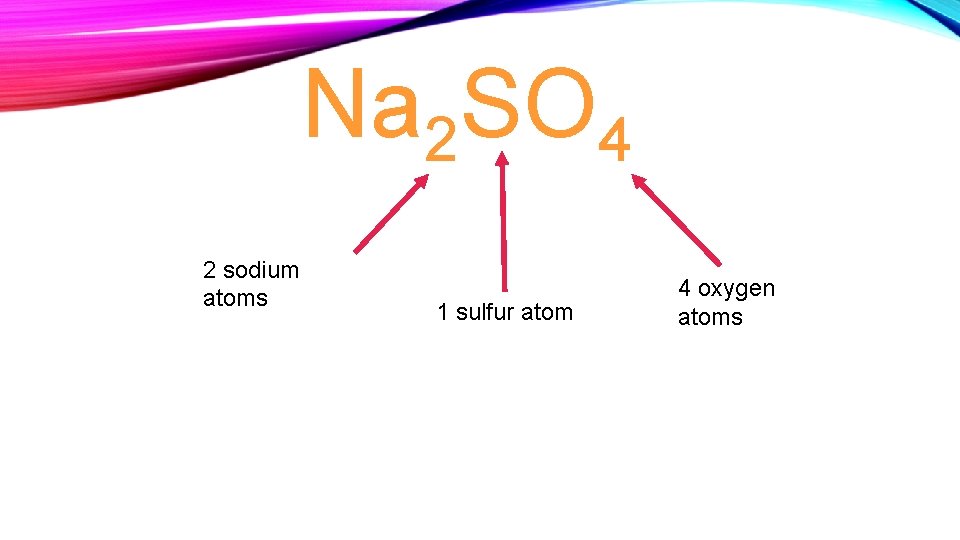
Na 2 SO 4 2 sodium atoms 1 sulfur atom 4 oxygen atoms
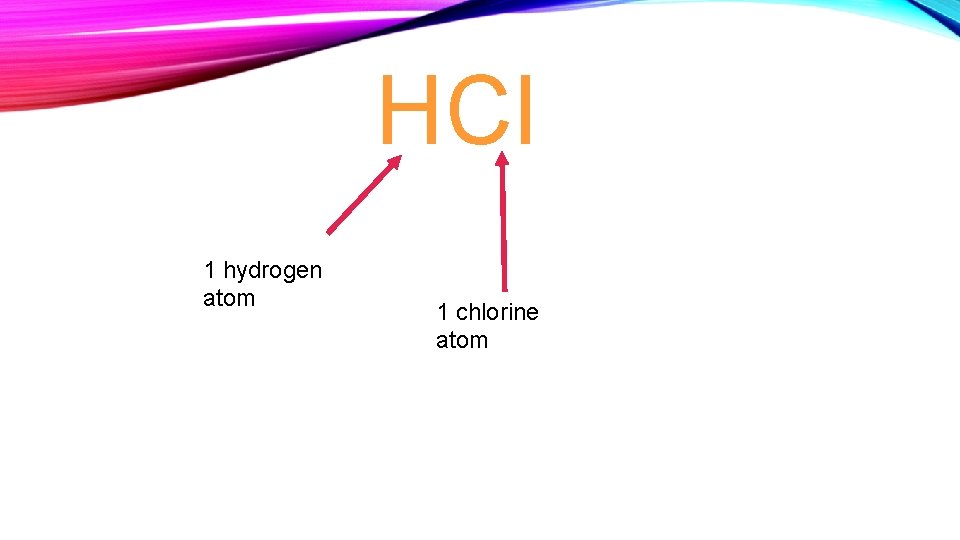
HCl 1 hydrogen atom 1 chlorine atom
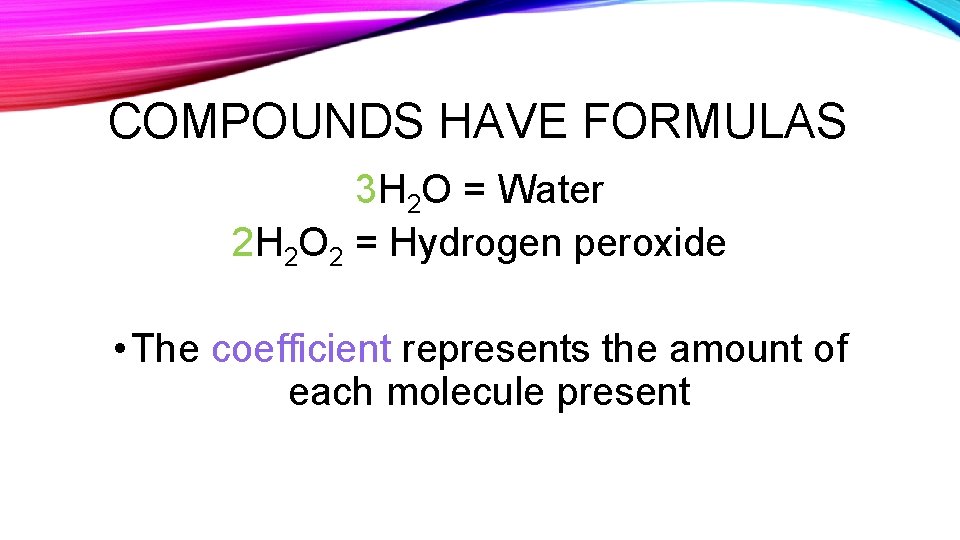
COMPOUNDS HAVE FORMULAS 3 H 2 O = Water 2 H 2 O 2 = Hydrogen peroxide • The coefficient represents the amount of each molecule present
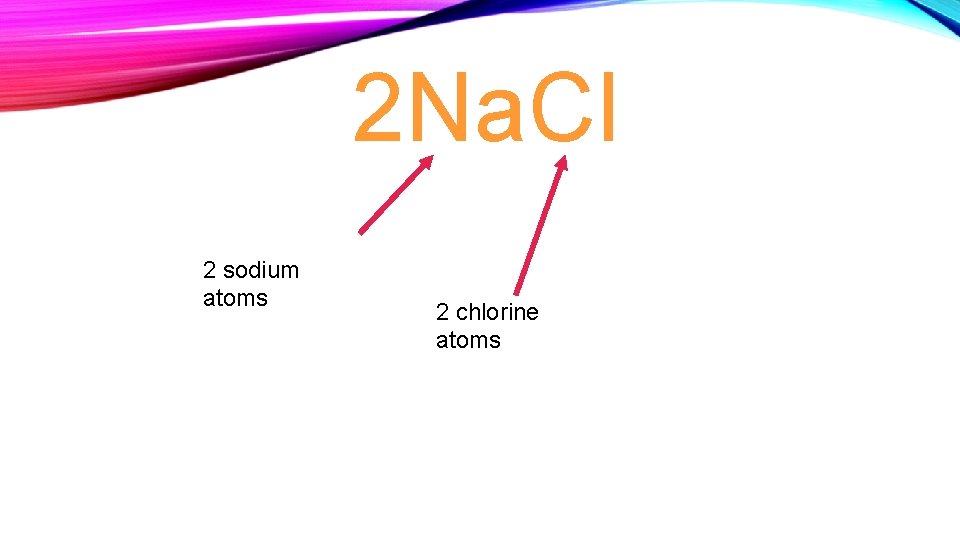
2 Na. Cl 2 sodium atoms 2 chlorine atoms

2 Na 2 SO 4 4 sodium atoms 2 sulfur atoms 8 oxygen atoms
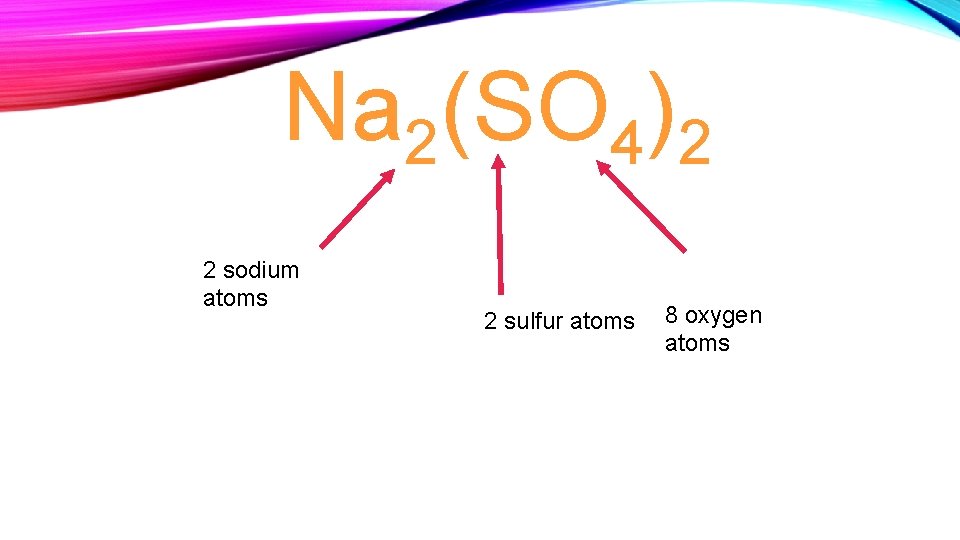
Na 2(SO 4)2 2 sodium atoms 2 sulfur atoms 8 oxygen atoms
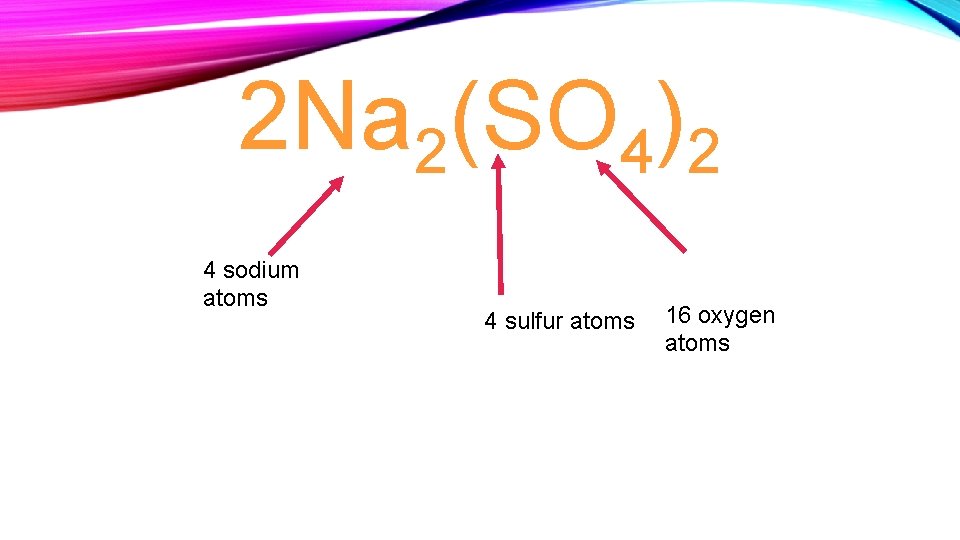
2 Na 2(SO 4)2 4 sodium atoms 4 sulfur atoms 16 oxygen atoms
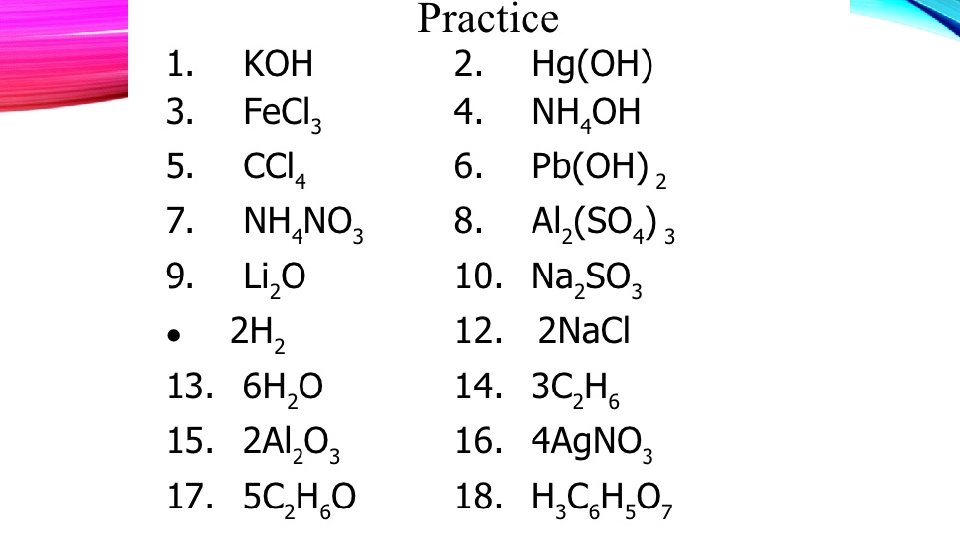

What is the Law of Conservation of Mass? Counting atoms due today! Adopt-an. Element projec due yesterday

BALANCING CHEMICAL EQUATIONS A chemical reaction is shown in as a chemical equation
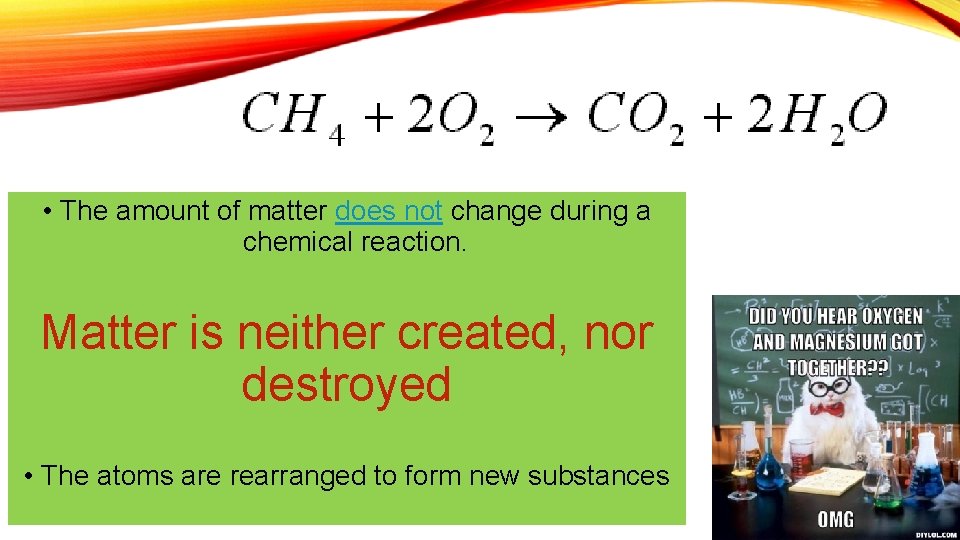
• The amount of matter does not change during a chemical reaction. Matter is neither created, nor destroyed • The atoms are rearranged to form new substances
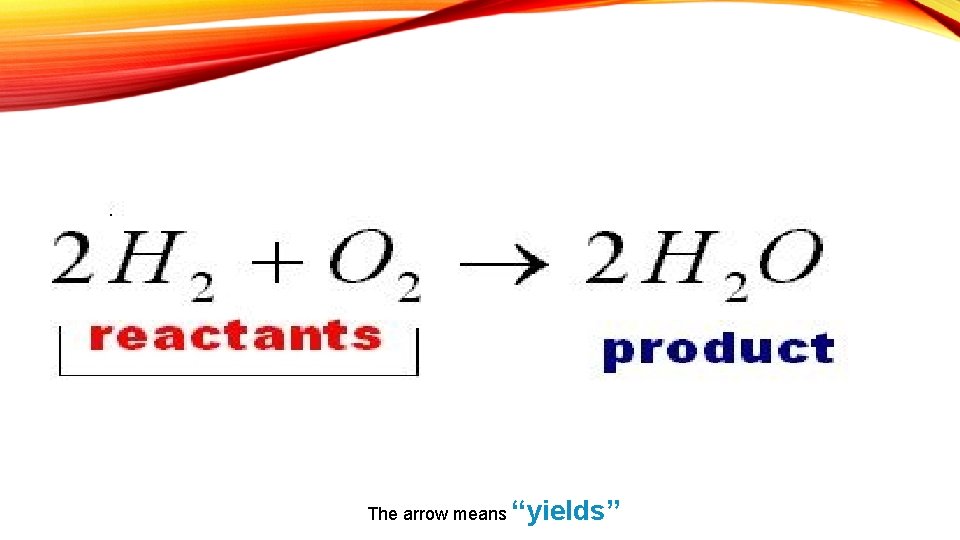
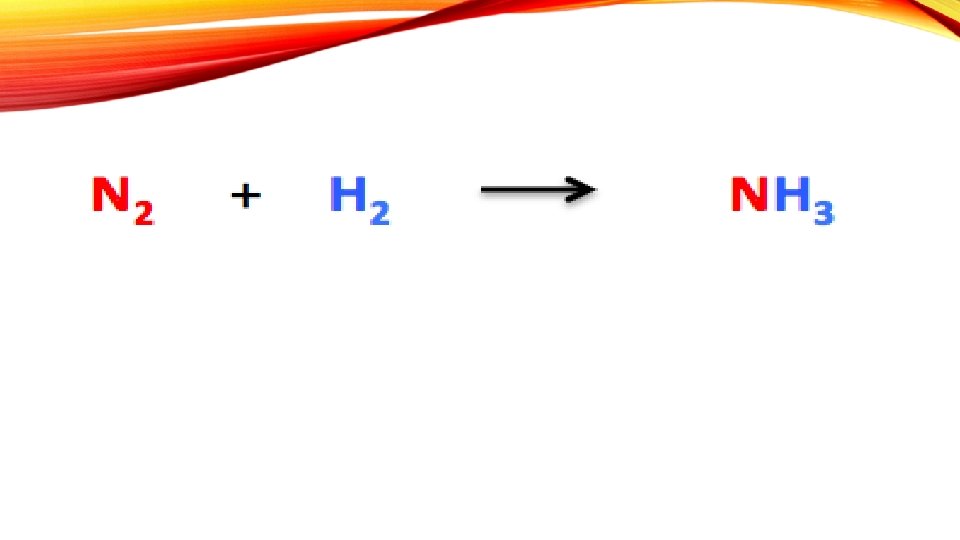
The arrow means “yields”
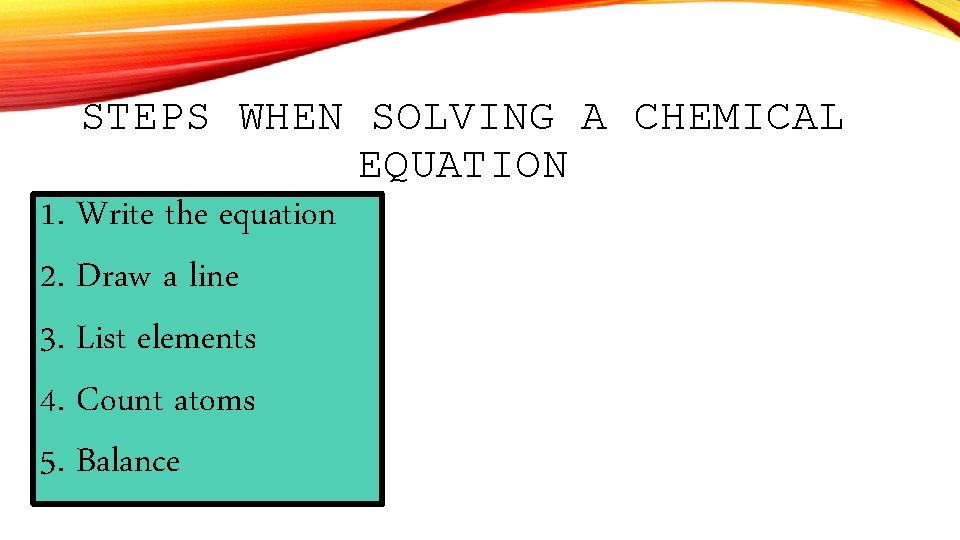
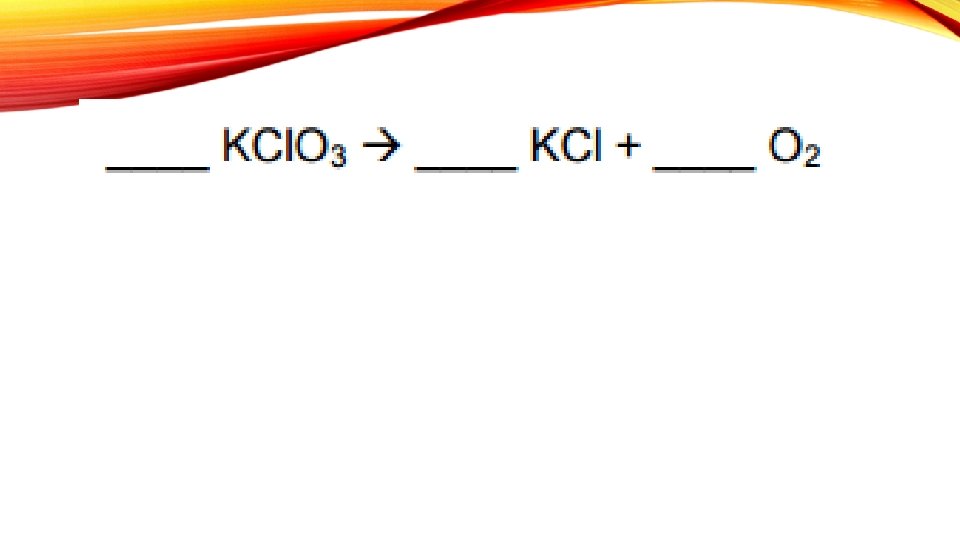
STEPS WHEN SOLVING A CHEMICAL EQUATION 1. Write the equation 2. Draw a line 3. List elements 4. Count atoms 5. Balance
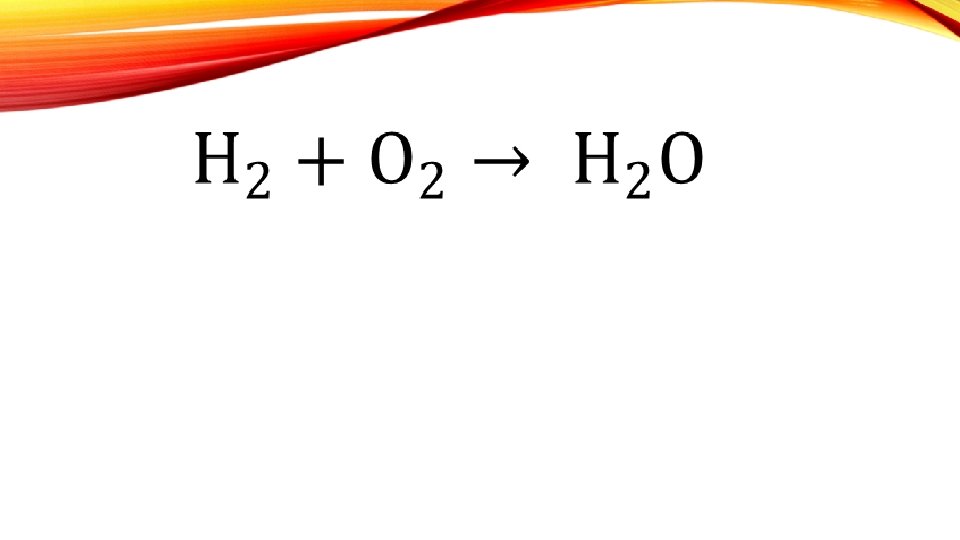
- Slides: 22