Models of Matter Atomic Structure OUTCOME QUESTIONS S
- Slides: 14

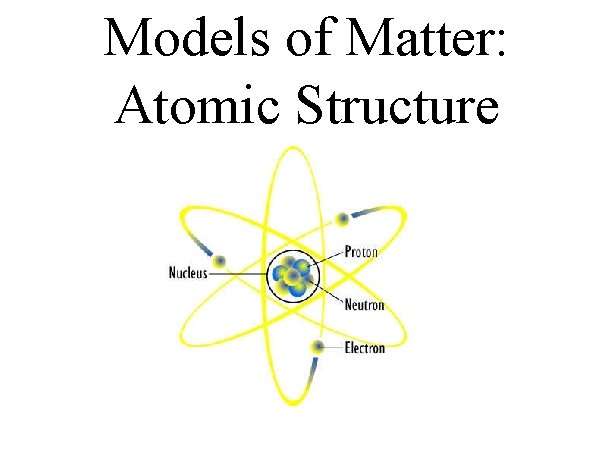
Models of Matter: Atomic Structure

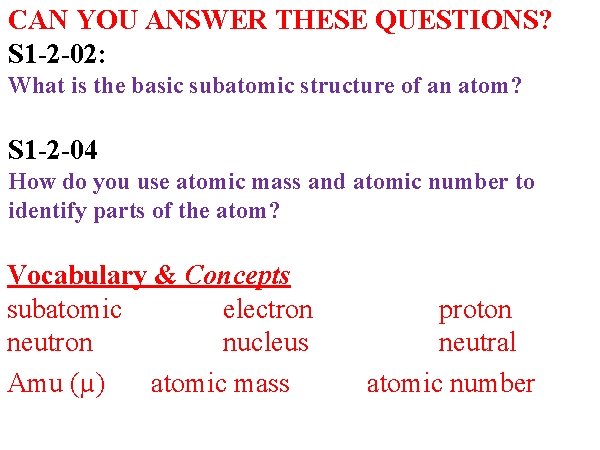
OUTCOME QUESTION(S): S 1 -2 -02: What is the basic subatomic structure of an atom? S 1 -2 -04 How do you use atomic mass and atomic number to identify parts of the atom? Vocabulary & Concepts subatomic electron neutron nucleus Amu (u) atomic mass proton neutral atomic number

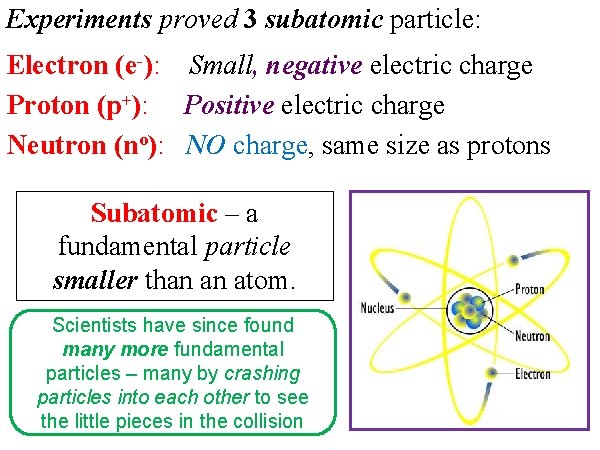
Experiments proved 3 subatomic particle: Electron (e-): Small, negative electric charge Proton (p+): Positive electric charge Neutron (no): NO charge, same size as protons Subatomic – a fundamental particle smaller than an atom. Scientists have since found many more fundamental particles – many by crashing particles into each other to see the little pieces in the collision

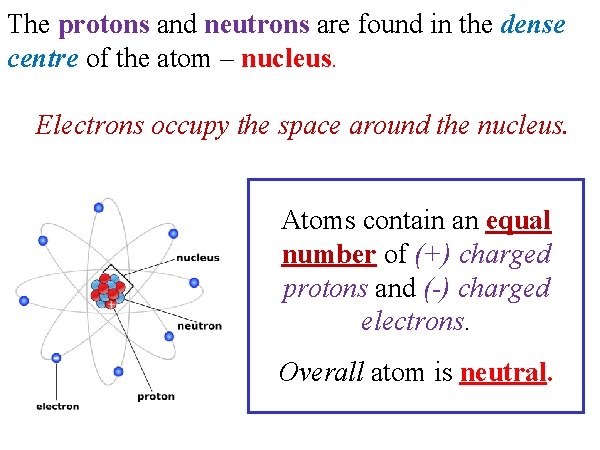
The protons and neutrons are found in the dense centre of the atom – nucleus. Electrons occupy the space around the nucleus. Atoms contain an equal number of (+) charged protons and (-) charged electrons. Overall atom is neutral.

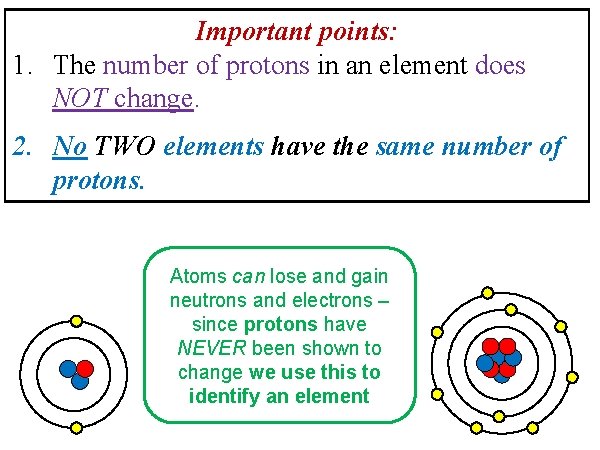
Important points: 1. The number of protons in an element does NOT change. 2. No TWO elements have the same number of protons. Atoms can lose and gain neutrons and electrons – since protons have NEVER been shown to change we use this to identify an element

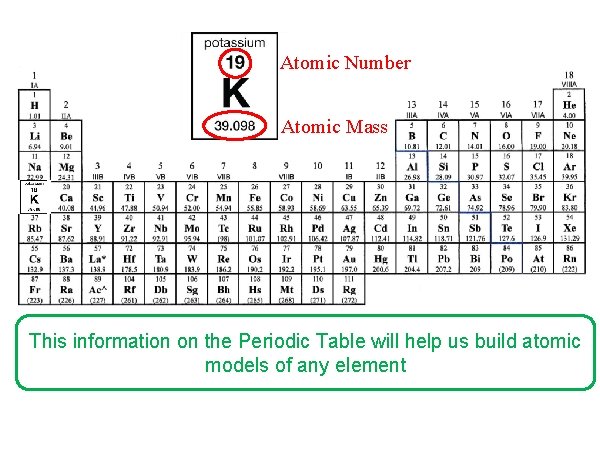
Atomic Number Atomic Mass This information on the Periodic Table will help us build atomic models of any element

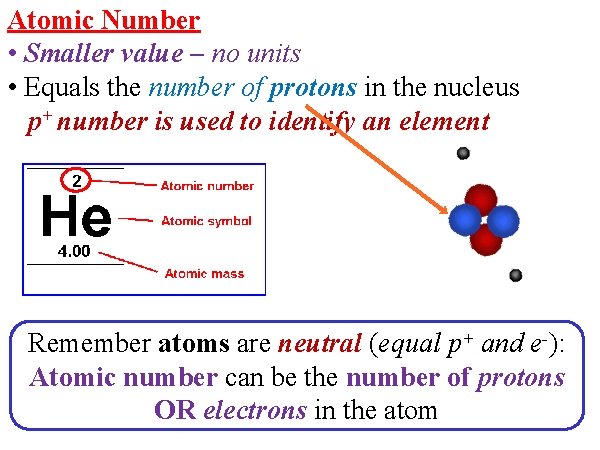
Atomic Number • Smaller value – no units • Equals the number of protons in the nucleus p+ number is used to identify an element Remember atoms are neutral (equal p+ and e-): Atomic number can be the number of protons OR electrons in the atom

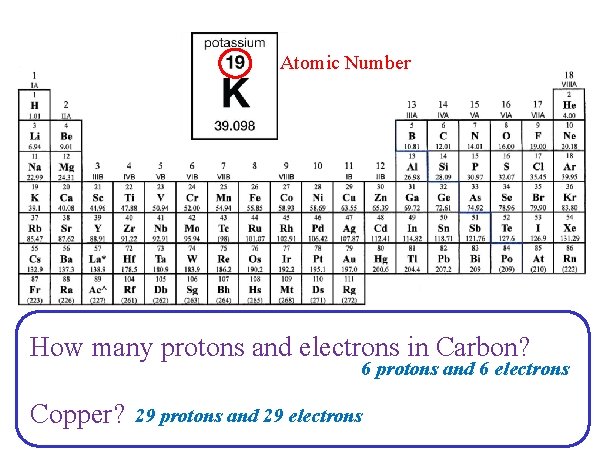
Atomic Number How many protons and electrons in Carbon? 6 protons and 6 electrons Copper? 29 protons and 29 electrons

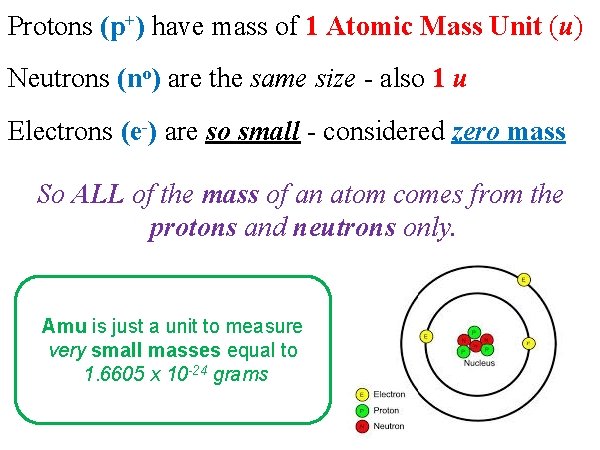
Protons (p+) have mass of 1 Atomic Mass Unit (u) Neutrons (no) are the same size - also 1 u Electrons (e-) are so small - considered zero mass So ALL of the mass of an atom comes from the protons and neutrons only. + Amu is just a unit to measure Protons Neutrons very small masses equal to 1. 6605 x 10 -24 grams - electrons

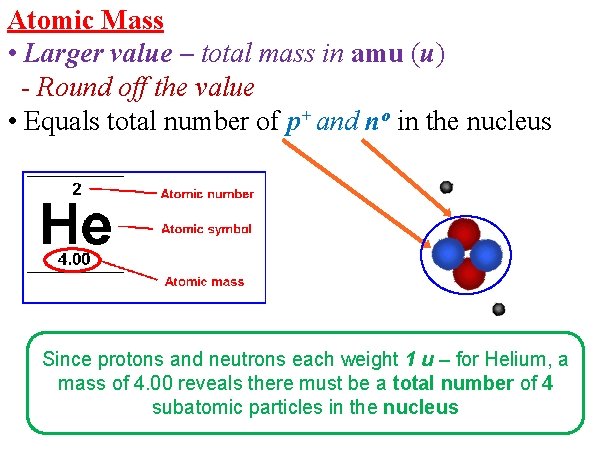
Atomic Mass • Larger value – total mass in amu (u) - Round off the value • Equals total number of p+ and no in the nucleus Since protons and neutrons each weight 1 u – for Helium, a mass of 4. 00 reveals there must be a total number of 4 subatomic particles in the nucleus

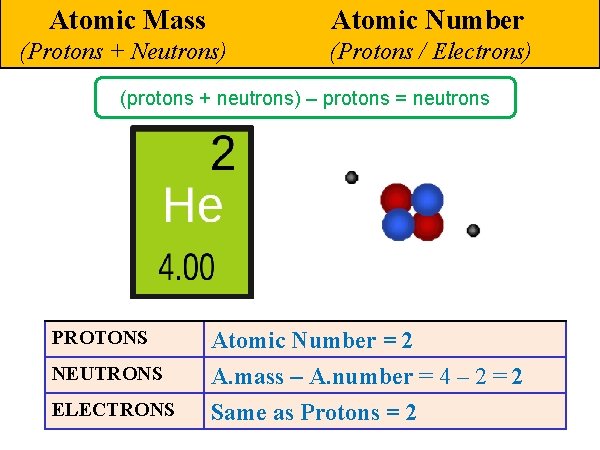
Atomic Mass Atomic Number (Protons + Neutrons) (Protons / Electrons) (protons + neutrons) – protons = neutrons PROTONS NEUTRONS ELECTRONS Atomic Number = 2 A. mass – A. number = 4 – 2 = 2 Same as Protons = 2

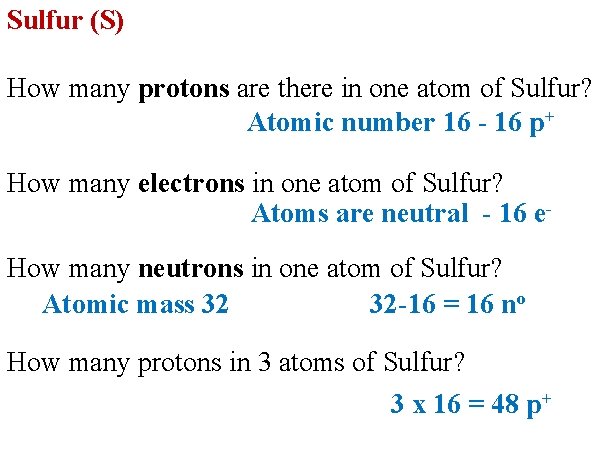
Sulfur (S) How many protons are there in one atom of Sulfur? Atomic number 16 - 16 p+ How many electrons in one atom of Sulfur? Atoms are neutral - 16 e- How many neutrons in one atom of Sulfur? Atomic mass 32 32 -16 = 16 no How many protons in 3 atoms of Sulfur? 3 x 16 = 48 p+

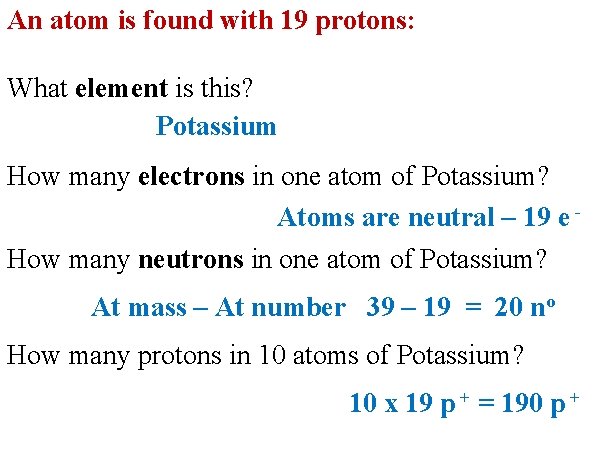
An atom is found with 19 protons: What element is this? Potassium How many electrons in one atom of Potassium? Atoms are neutral – 19 e How many neutrons in one atom of Potassium? At mass – At number 39 – 19 = 20 no How many protons in 10 atoms of Potassium? 10 x 19 p + = 190 p +

CAN YOU ANSWER THESE QUESTIONS? S 1 -2 -02: What is the basic subatomic structure of an atom? S 1 -2 -04 How do you use atomic mass and atomic number to identify parts of the atom? Vocabulary & Concepts subatomic electron neutron nucleus Amu (µ) atomic mass proton neutral atomic number