Do Now Draw a model of an atom






- Slides: 17

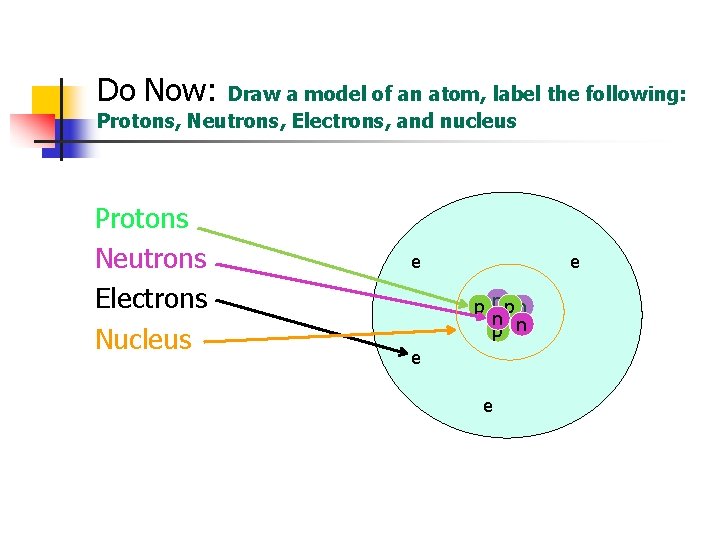
Do Now: Draw a model of an atom, label the following: Protons, Neutrons, Electrons, and nucleus Protons Neutrons Electrons Nucleus e e p npn n n p e e

HOMEWORK AND ANNOUNCEMENTS Due Today: Vocabulary, textbook, Research Plan, Forms 1 A and 1 B due today Science Fair Countdown Start collecting data! Chapter 9 textbook pages 322 -327 Vocabulary Word Find USA Test. Prep

Using the Periodic Table L. O. SWBAT use the periodic table to determine the number of p+ and e- in an atom.


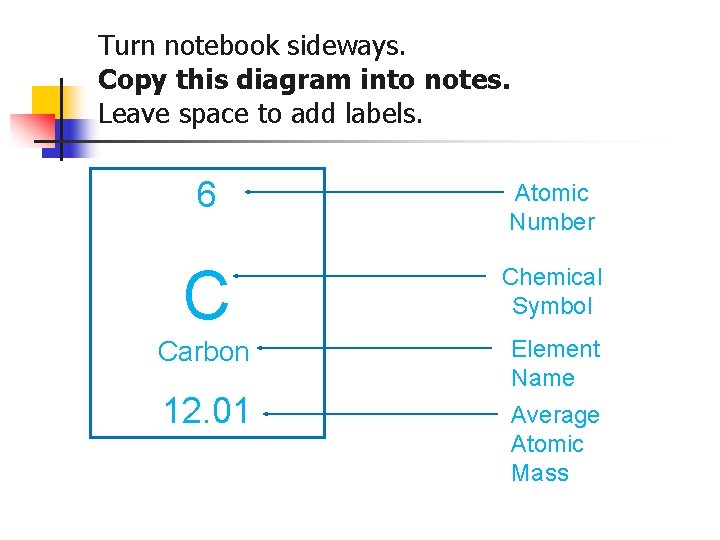
Turn notebook sideways. Copy this diagram into notes. Leave space to add labels. 6 Atomic Number C Chemical Symbol Carbon Element Name 12. 01 Average Atomic Mass

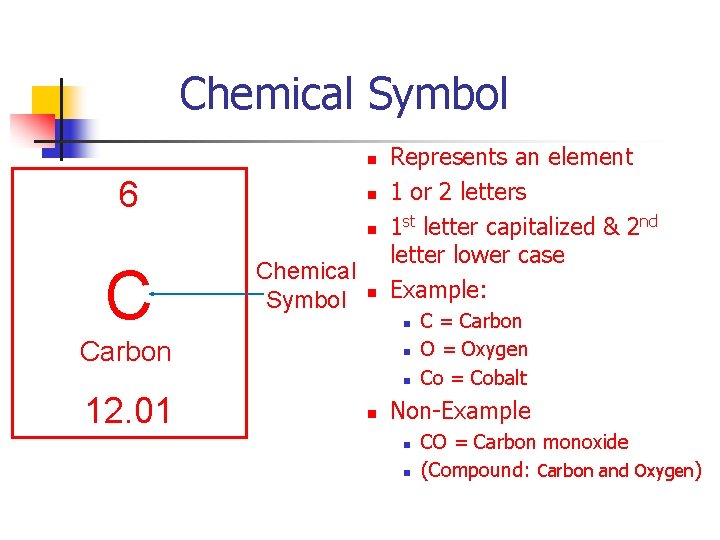
Chemical Symbol n 6 n n C Chemical Symbol n Represents an element 1 or 2 letters 1 st letter capitalized & 2 nd letter lower case Example: n Carbon n n 12. 01 n C = Carbon O = Oxygen Co = Cobalt Non-Example n n CO = Carbon monoxide (Compound: Carbon and Oxygen)

Use your periodic table to find the chemical symbol for the following elements. (Write the element and your answer in your notes) 1. Oxygen O 6. Chlorine Cl 2. Lithium Li 7. Phosphorus P 8. Aluminum Al 3. Beryllium Be 4. Potassium K 9. Silver Ag 5. Argon Ar 10. Tin Sn

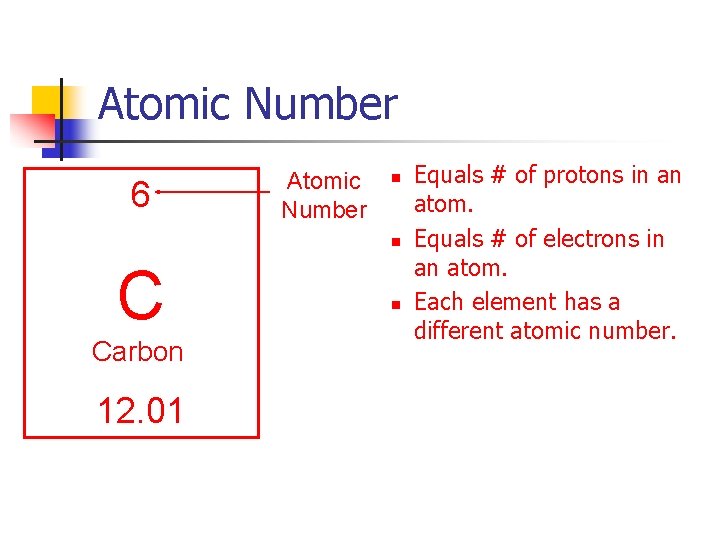
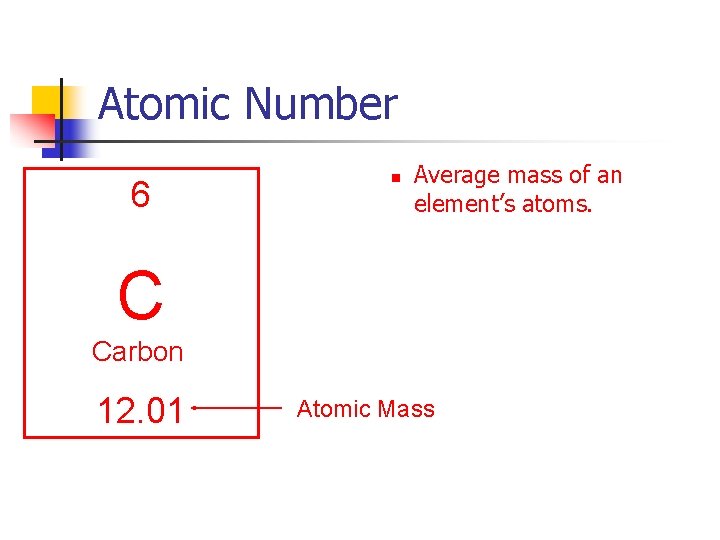
Atomic Number 6 Atomic Number n n C Carbon 12. 01 n Equals # of protons in an atom. Equals # of electrons in an atom. Each element has a different atomic number.

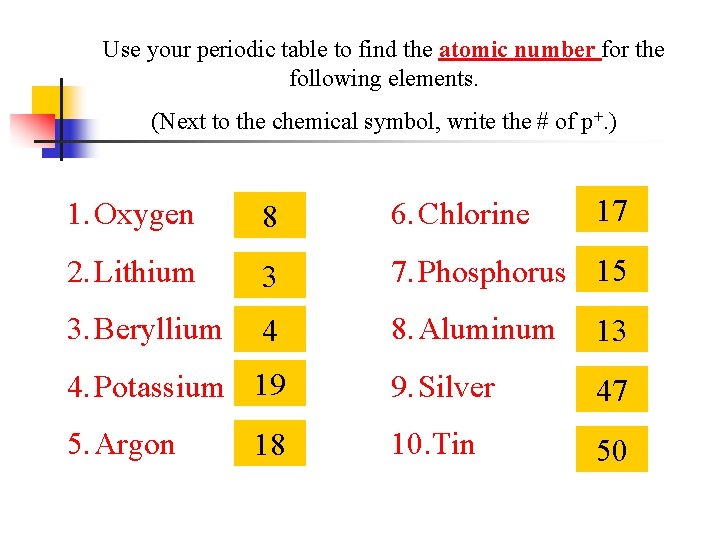
Use your periodic table to find the atomic number for the following elements. (Next to the chemical symbol, write the # of p+. ) 17 1. Oxygen 8 6. Chlorine 2. Lithium 3 7. Phosphorus 15 3. Beryllium 4 8. Aluminum 13 4. Potassium 19 9. Silver 47 5. Argon 10. Tin 50 18

Atomic Number 6 n Average mass of an element’s atoms. C Carbon 12. 01 Atomic Mass

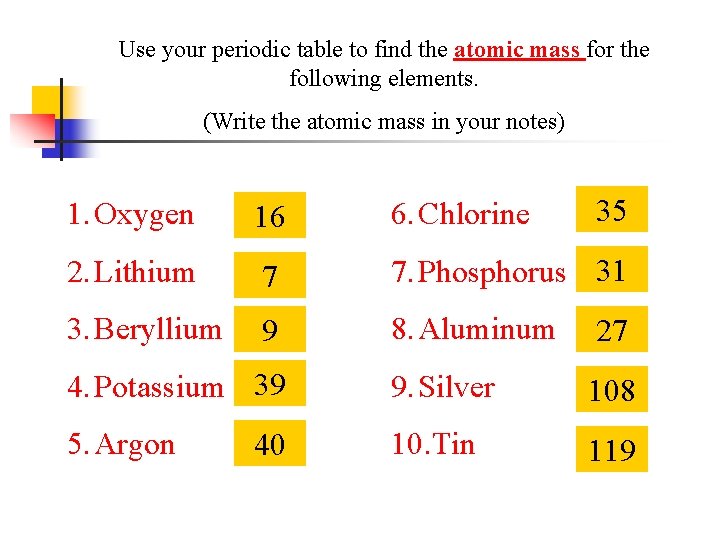
Use your periodic table to find the atomic mass for the following elements. (Write the atomic mass in your notes) 35 1. Oxygen 16 6. Chlorine 2. Lithium 7 7. Phosphorus 31 3. Beryllium 9 8. Aluminum 27 4. Potassium 39 9. Silver 108 5. Argon 10. Tin 119 40

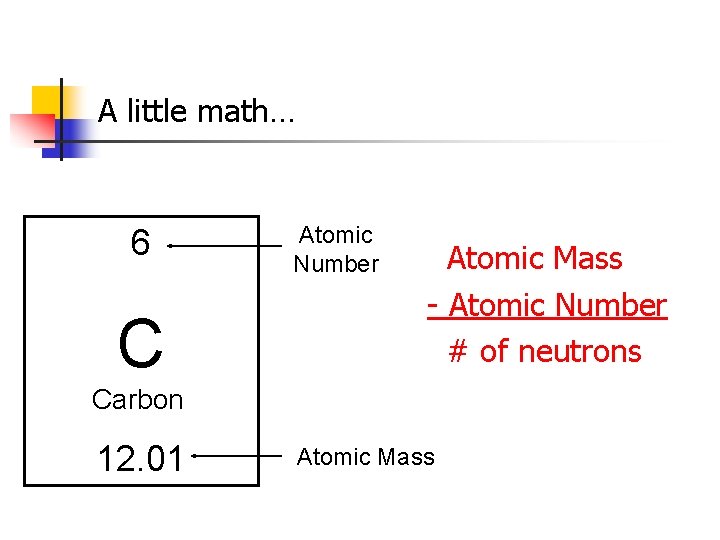
A little math… 6 C Atomic Number Atomic Mass - Atomic Number # of neutrons Carbon 12. 01 Atomic Mass

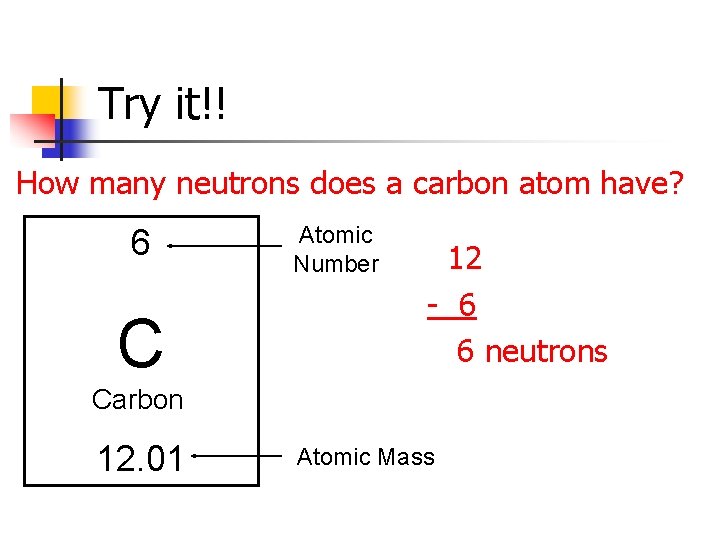
Try it!! How many neutrons does a carbon atom have? 6 C Atomic Number 12 - 6 6 neutrons Carbon 12. 01 Atomic Mass

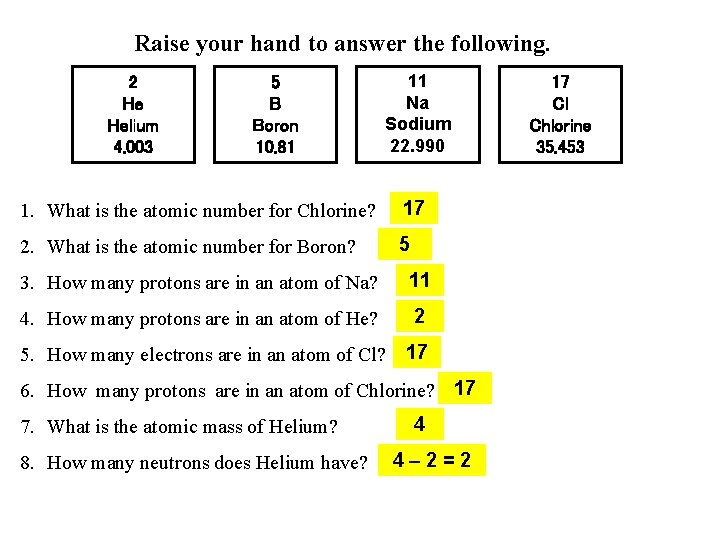
Raise your hand to answer the following. 2 He Helium 4. 003 5 B Boron 10. 81 11 Na Sodium 22. 990 1. What is the atomic number for Chlorine? 17 2. What is the atomic number for Boron? 5 3. How many protons are in an atom of Na? 11 4. How many protons are in an atom of He? 2 5. How many electrons are in an atom of Cl? 17 6. How many protons are in an atom of Chlorine? 17 7. What is the atomic mass of Helium? 8. How many neutrons does Helium have? 4 4– 2=2 17 Cl Chlorine 35. 453

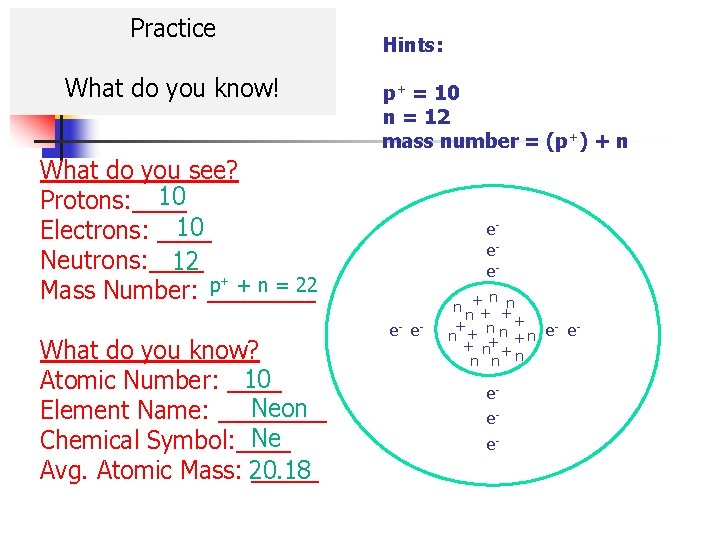
Practice What do you know! Hints: p+ = 10 n = 12 mass number = (p+) + n What do you see? 10 Protons: ____ 10 Electrons: ____ Neutrons: ____ 12 p+ + n = 22 Mass Number: ____ What do you know? 10 Atomic Number: ____ Neon Element Name: ____ Ne Chemical Symbol: ____ Avg. Atomic Mass: 20. 18 _____ eee+ n n n+ + n n + e- en n + n+++ n n e- e- n n eee-

Summary (3 -4 sentences) Today I learned about… Helper questions How do we determine the number of p+ an atom has? Electrons? Netrons?

Independent Work n Test Corrections – in notebook n n n Question, correct answer, and why Textbook Pages 322 -327 Vocabulary Word Find n definitions (in notebook)