Protons Identify an Elementatom 2 protons Helium Protons


- Slides: 16


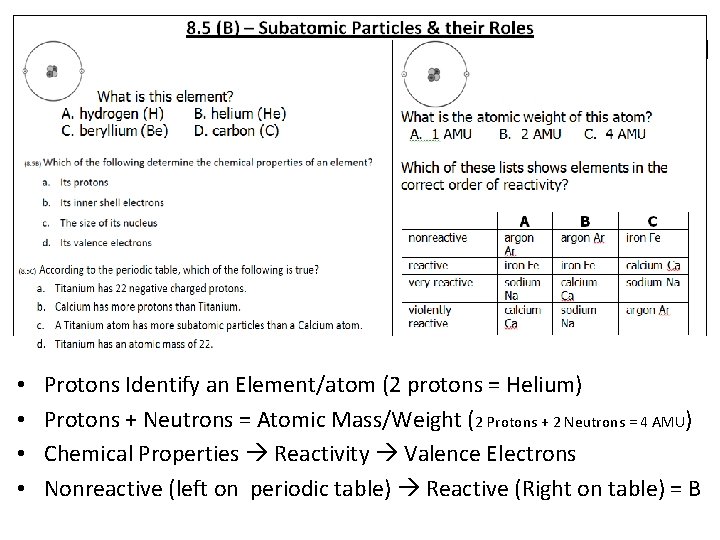
• • Protons Identify an Element/atom (2 protons = Helium) Protons + Neutrons = Atomic Mass/Weight (2 Protons + 2 Neutrons = 4 AMU) Chemical Properties Reactivity Valence Electrons Nonreactive (left on periodic table) Reactive (Right on table) = B

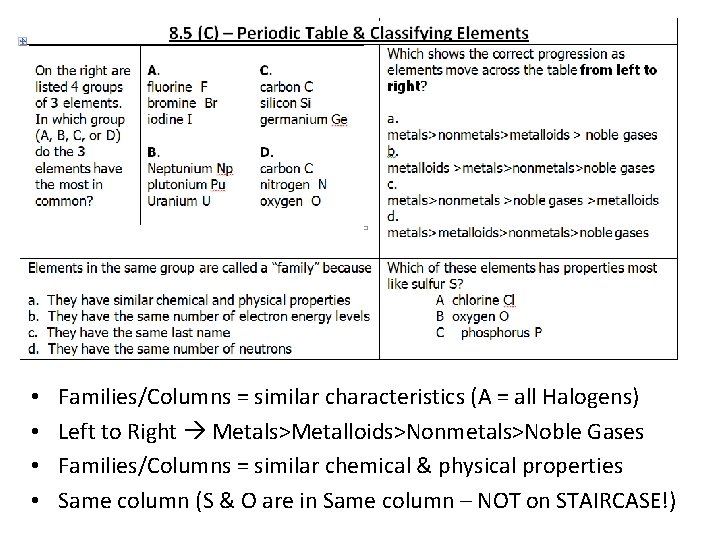
• • Families/Columns = similar characteristics (A = all Halogens) Left to Right Metals>Metalloids>Nonmetals>Noble Gases Families/Columns = similar chemical & physical properties Same column (S & O are in Same column – NOT on STAIRCASE!)

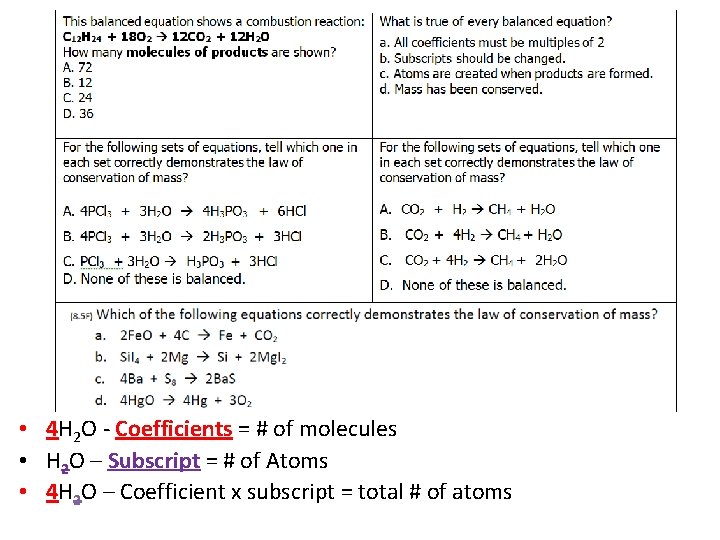
• 4 H 2 O - Coefficients = # of molecules • H 2 O – Subscript = # of Atoms • 4 H 2 O – Coefficient x subscript = total # of atoms

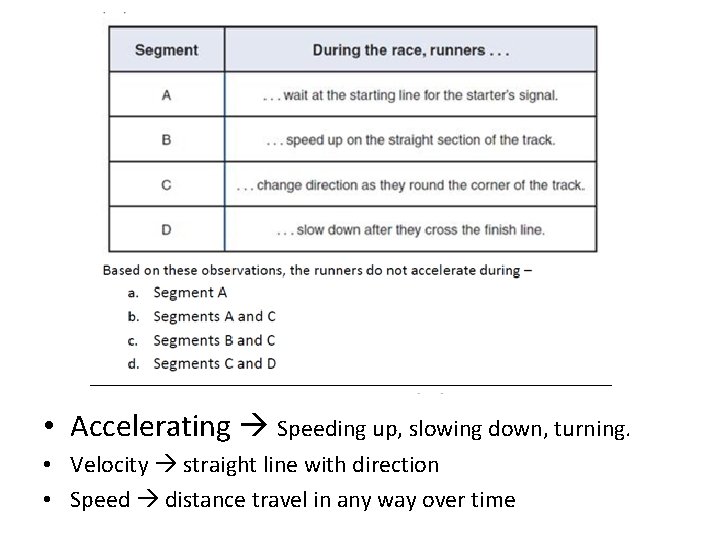
• Accelerating Speeding up, slowing down, turning. • Velocity straight line with direction • Speed distance travel in any way over time

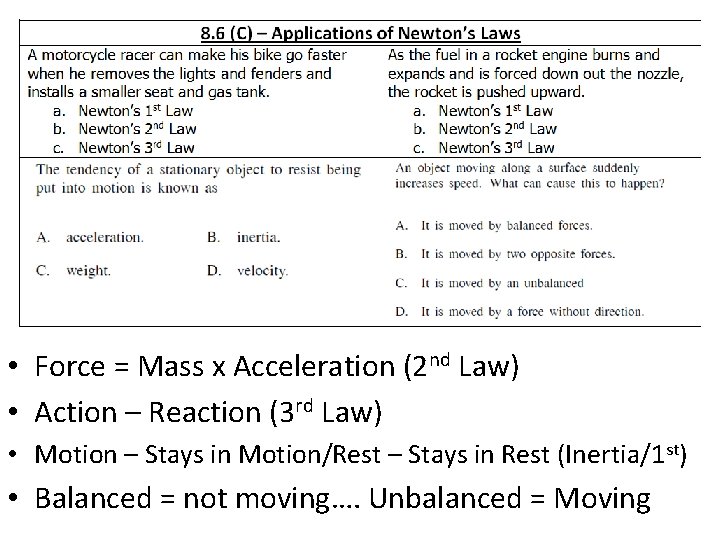
• Force = Mass x Acceleration (2 nd Law) • Action – Reaction (3 rd Law) • Motion – Stays in Motion/Rest – Stays in Rest (Inertia/1 st) • Balanced = not moving…. Unbalanced = Moving

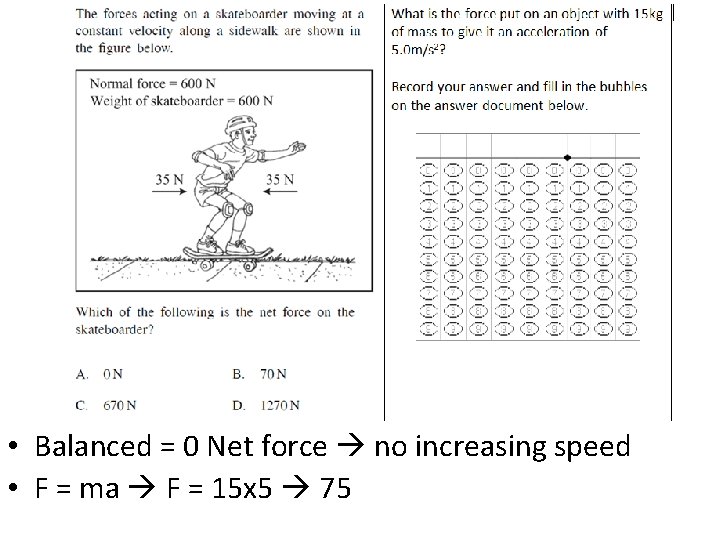
• Balanced = 0 Net force no increasing speed • F = ma F = 15 x 5 75

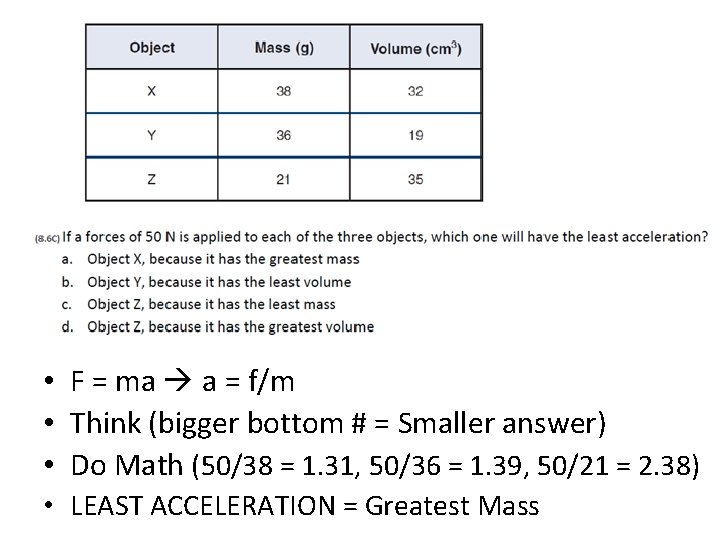
• F = ma a = f/m • Think (bigger bottom # = Smaller answer) • Do Math (50/38 = 1. 31, 50/36 = 1. 39, 50/21 = 2. 38) • LEAST ACCELERATION = Greatest Mass

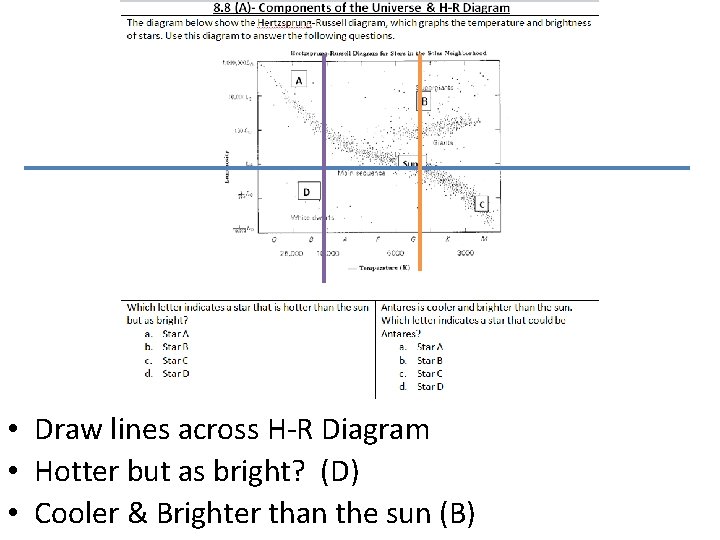
• Draw lines across H-R Diagram • Hotter but as bright? (D) • Cooler & Brighter than the sun (B)

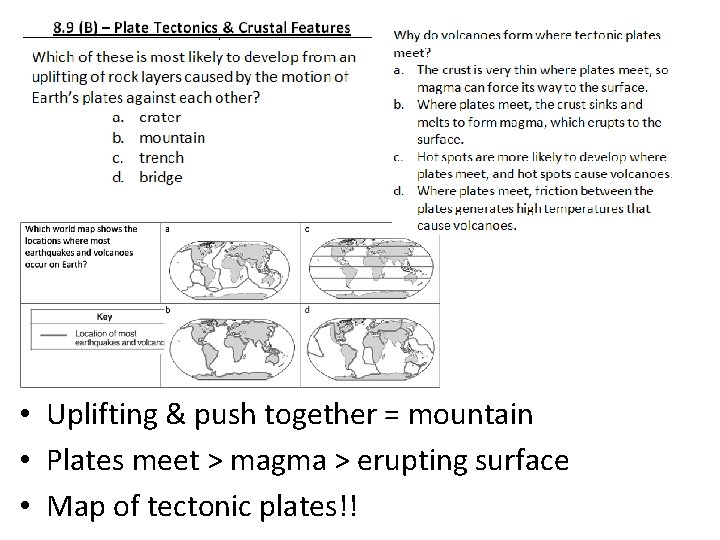
• Uplifting & push together = mountain • Plates meet > magma > erupting surface • Map of tectonic plates!!

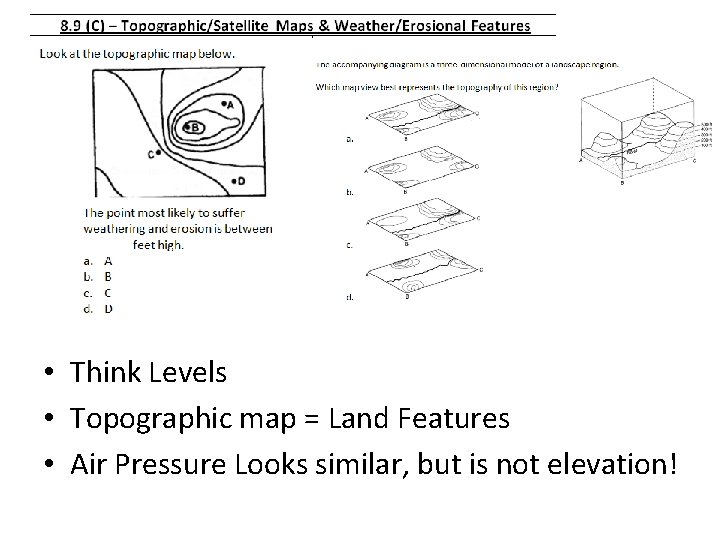
• Think Levels • Topographic map = Land Features • Air Pressure Looks similar, but is not elevation!

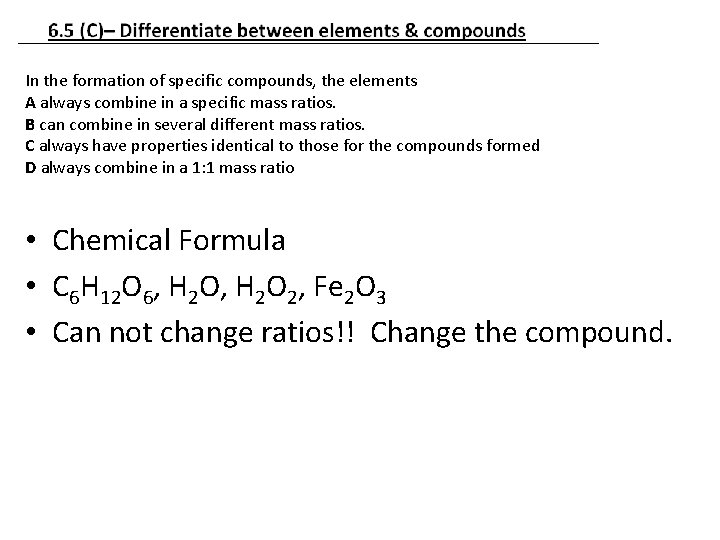
In the formation of specific compounds, the elements A always combine in a specific mass ratios. B can combine in several different mass ratios. C always have properties identical to those for the compounds formed D always combine in a 1: 1 mass ratio • Chemical Formula • C 6 H 12 O 6, H 2 O 2, Fe 2 O 3 • Can not change ratios!! Change the compound.

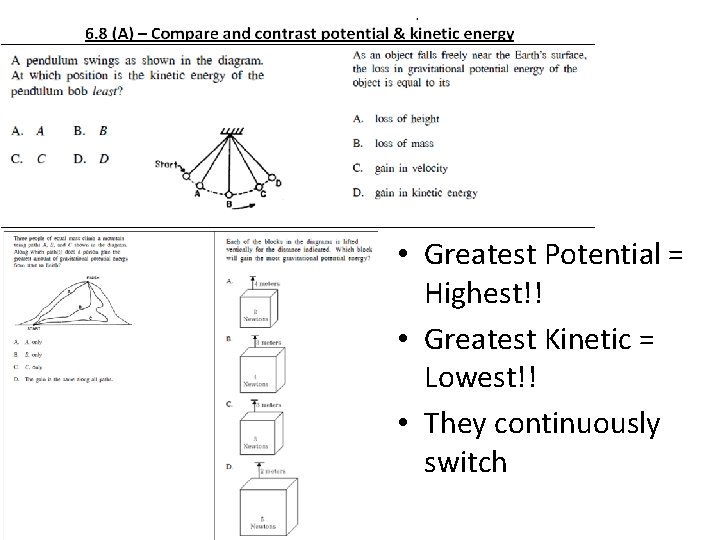
• Greatest Potential = Highest!! • Greatest Kinetic = Lowest!! • They continuously switch

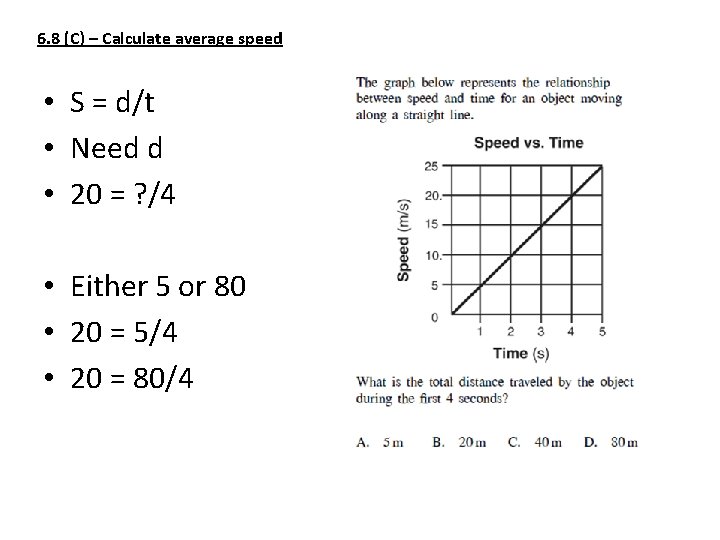
6. 8 (C) – Calculate average speed • S = d/t • Need d • 20 = ? /4 • Either 5 or 80 • 20 = 5/4 • 20 = 80/4

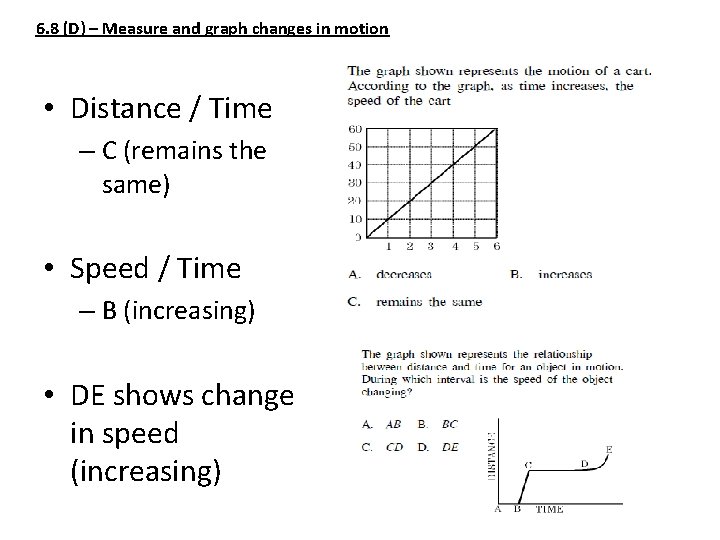
6. 8 (D) – Measure and graph changes in motion • Distance / Time – C (remains the same) • Speed / Time – B (increasing) • DE shows change in speed (increasing)
