Lecture 20 Helium and heavier atoms Helium and





- Slides: 24

Lecture 20 Helium and heavier atoms

Helium and heavier atoms l l l We use the exact solutions of hydrogenic Schrödinger equation or orbitals to construct an approximate wave function of a manyelectron atom, the helium and heavier atoms. Unlike the hydrogenic atom, the discussion here is approximate and some rules introduced can have exceptions. Spins and antisymmetry of fermion wave functions start to play a critical role.

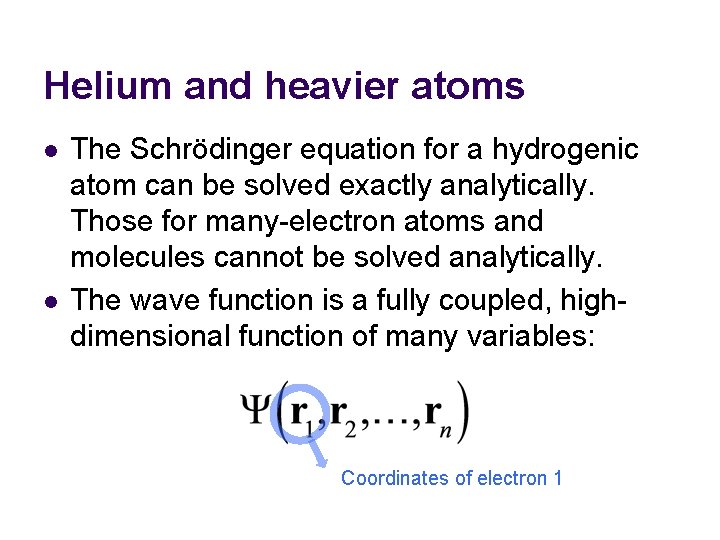
Helium and heavier atoms l l The Schrödinger equation for a hydrogenic atom can be solved exactly analytically. Those for many-electron atoms and molecules cannot be solved analytically. The wave function is a fully coupled, highdimensional function of many variables: Coordinates of electron 1

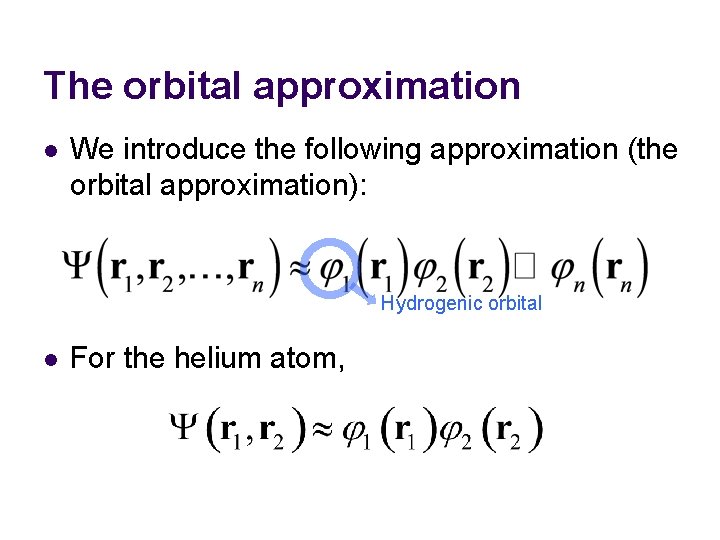
The orbital approximation l We introduce the following approximation (the orbital approximation): Hydrogenic orbital l For the helium atom,

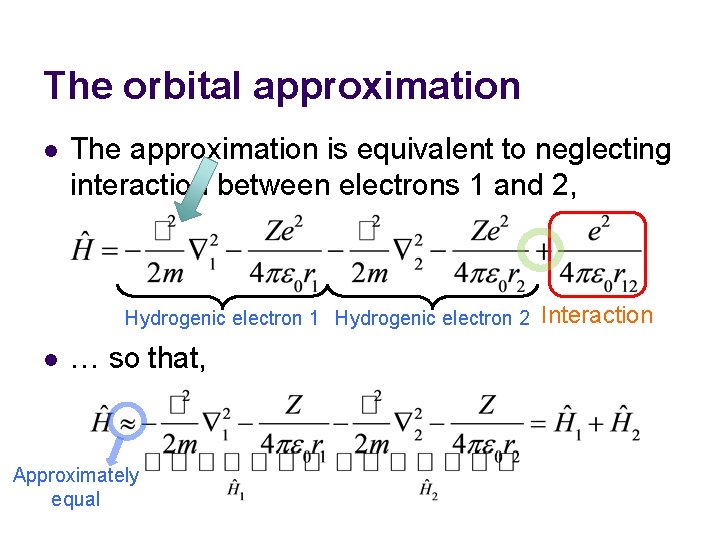
The orbital approximation l The approximation is equivalent to neglecting interaction between electrons 1 and 2, Hydrogenic electron 1 Hydrogenic electron 2 l … so that, Approximately equal Interaction

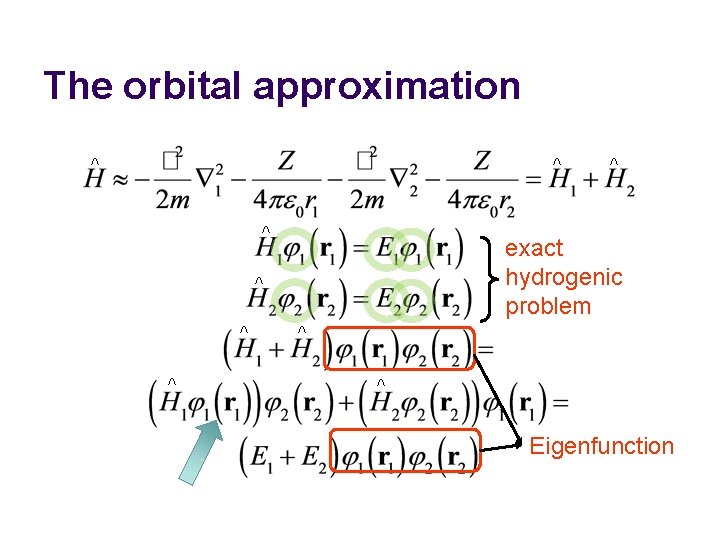
The orbital approximation ^ ^ ^ exact hydrogenic problem ^ ^ ^ Eigenfunction

The orbital approximation l We construct a helium wave function as the product of hydrogenic orbitals with Z = 2. l Issue #1: an electron is fermion and fermions’ wave function must be antisymmetric with respect to interchange (the above isn’t): l Issue #2: each electron must be either spin α or β (the above neglects spins).

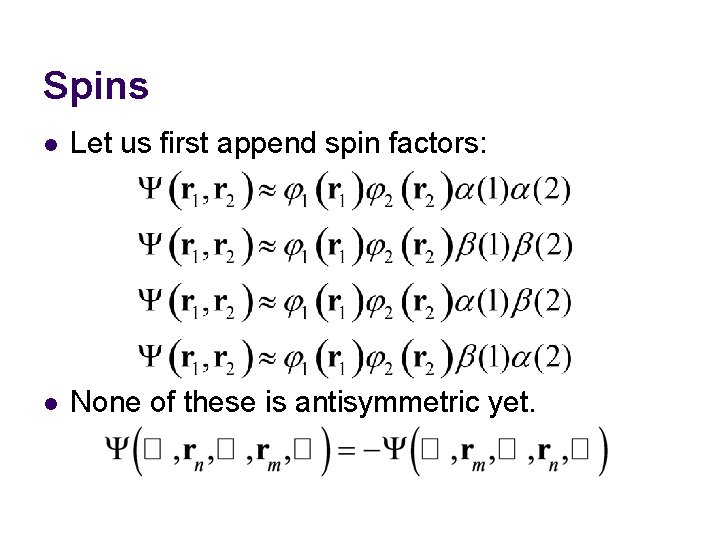
Spins l Let us first append spin factors: l None of these is antisymmetric yet.

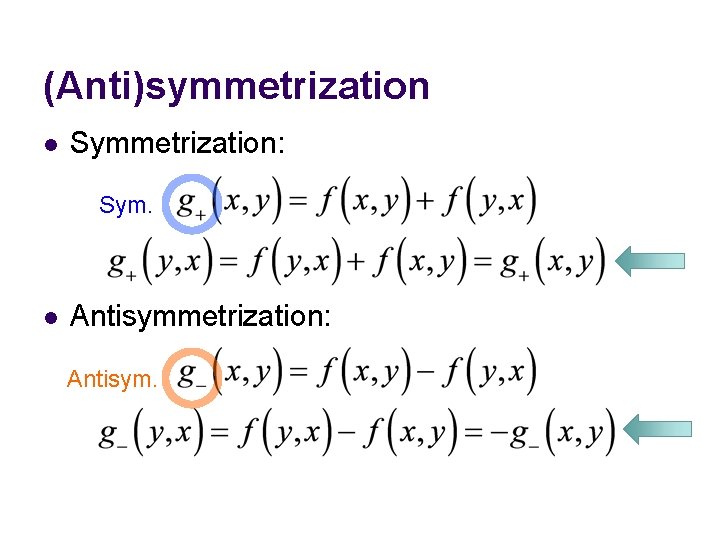
(Anti)symmetrization l Symmetrization: Sym. l Antisymmetrization: Antisym.

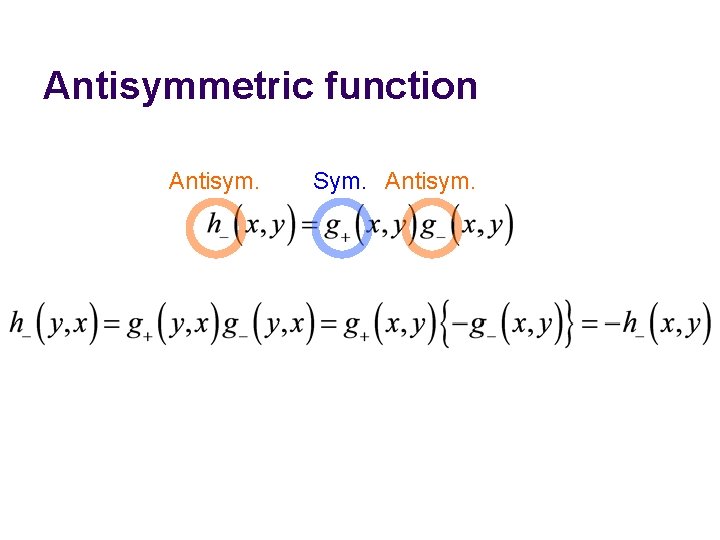
Antisymmetric function Antisym. Sym. Antisym.

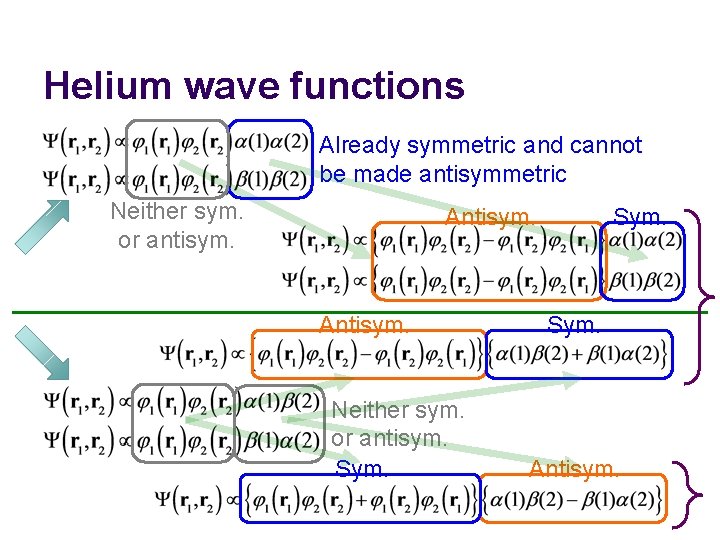
Helium wave functions Already symmetric and cannot be made antisymmetric Neither sym. or antisym. Antisym. Neither sym. or antisym. Sym. Antisym.

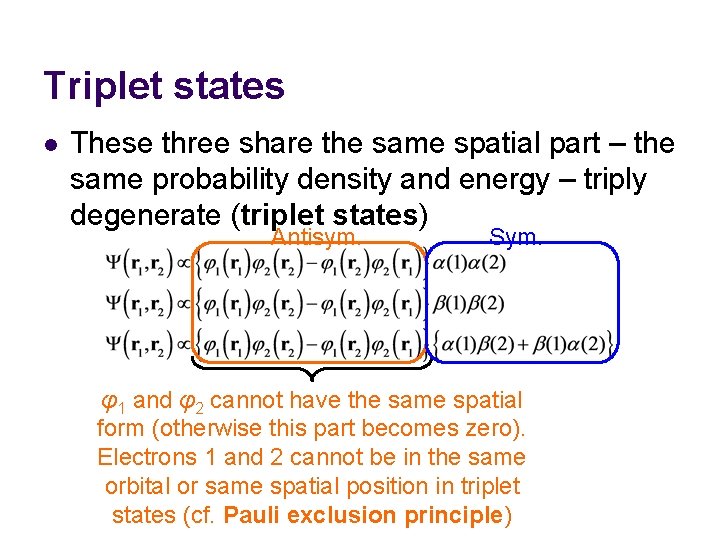
Triplet states l These three share the same spatial part – the same probability density and energy – triply degenerate (triplet states) Antisym. Sym. φ1 and φ2 cannot have the same spatial form (otherwise this part becomes zero). Electrons 1 and 2 cannot be in the same orbital or same spatial position in triplet states (cf. Pauli exclusion principle)

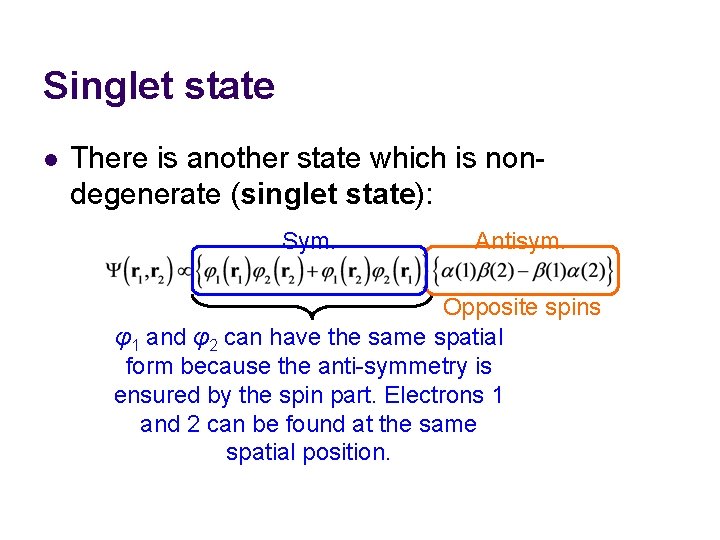
Singlet state l There is another state which is nondegenerate (singlet state): Sym. Antisym. Opposite spins φ1 and φ2 can have the same spatial form because the anti-symmetry is ensured by the spin part. Electrons 1 and 2 can be found at the same spatial position.

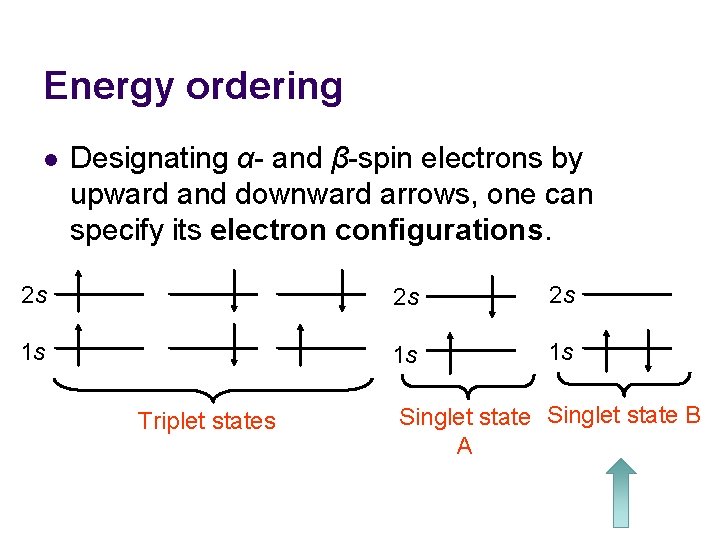
Energy ordering l Designating α- and β-spin electrons by upward and downward arrows, one can specify its electron configurations. 2 s 2 s 2 s 1 s 1 s 1 s Triplet states Singlet state B A

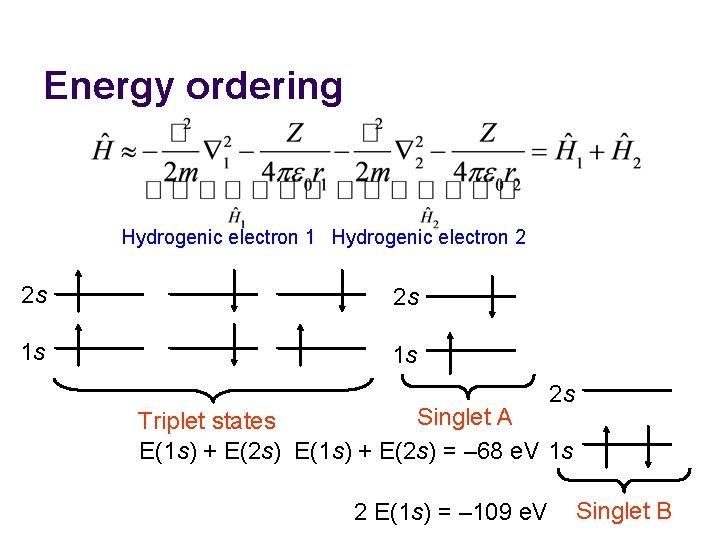
Energy ordering Hydrogenic electron 1 Hydrogenic electron 2 2 s 2 s 1 s 1 s 2 s Singlet A Triplet states E(1 s) + E(2 s) = – 68 e. V 1 s 2 E(1 s) = – 109 e. V Singlet B

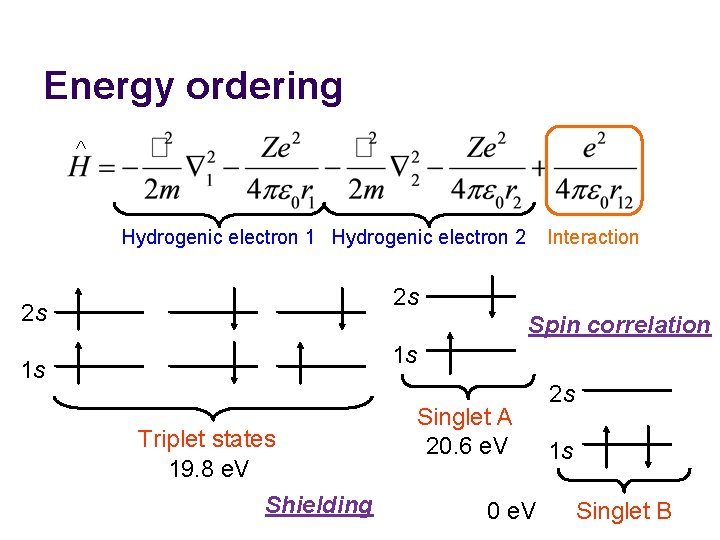
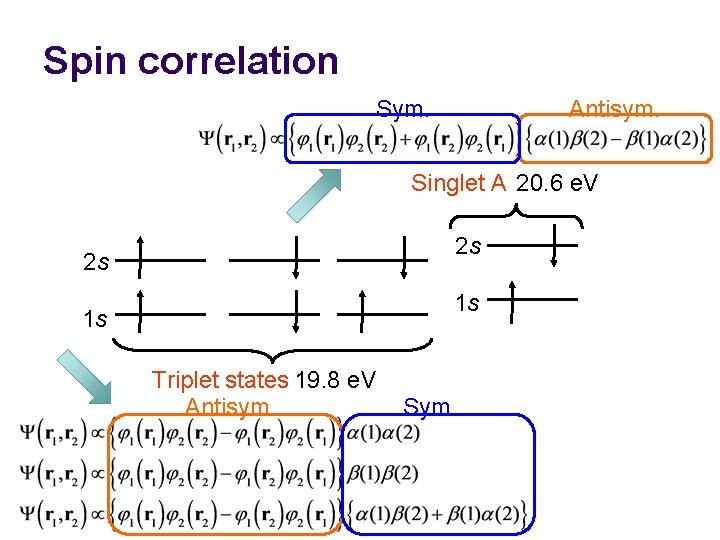
Energy ordering ^ Interaction Hydrogenic electron 1 Hydrogenic electron 2 2 s 2 s Spin correlation 1 s 1 s Triplet states 19. 8 e. V Shielding Singlet A 20. 6 e. V 0 e. V 2 s 1 s Singlet B

Spin correlation Sym. Antisym. Singlet A 20. 6 e. V 2 s 2 s 1 s 1 s Triplet states 19. 8 e. V Antisym. Sym.

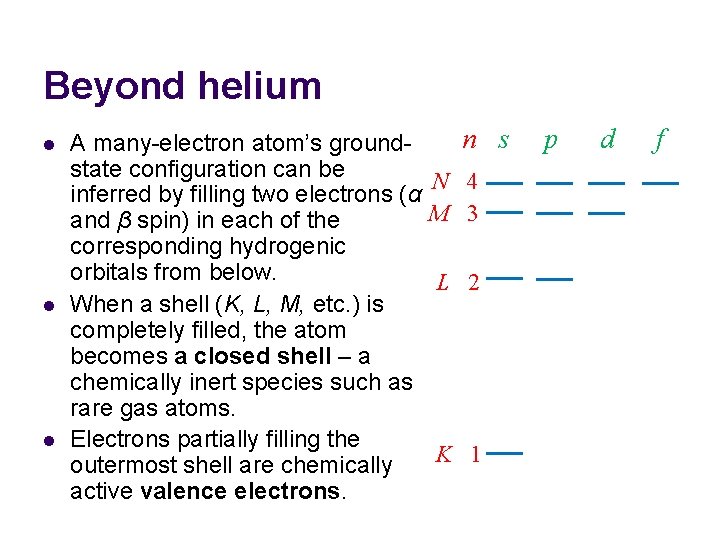
Beyond helium l l l A many-electron atom’s groundstate configuration can be inferred by filling two electrons (α and β spin) in each of the corresponding hydrogenic orbitals from below. When a shell (K, L, M, etc. ) is completely filled, the atom becomes a closed shell – a chemically inert species such as rare gas atoms. Electrons partially filling the outermost shell are chemically active valence electrons. n s N 4 M 3 L 2 K 1 p d f

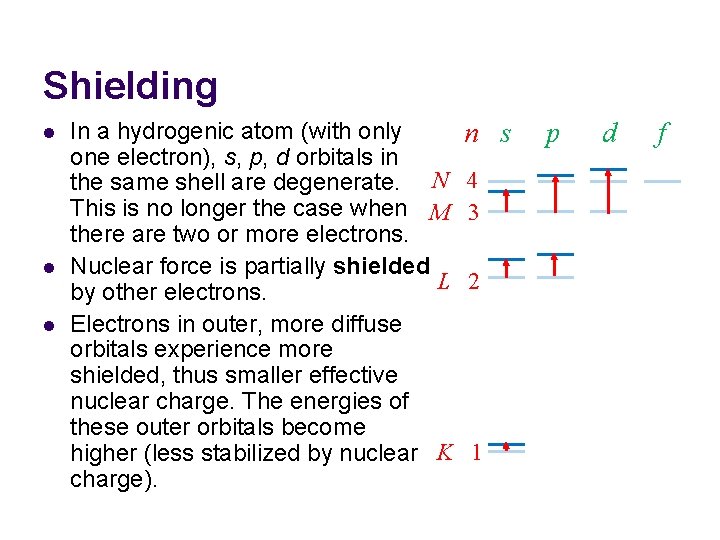
Shielding l l l In a hydrogenic atom (with only one electron), s, p, d orbitals in the same shell are degenerate. N This is no longer the case when M there are two or more electrons. Nuclear force is partially shielded L by other electrons. Electrons in outer, more diffuse orbitals experience more shielded, thus smaller effective nuclear charge. The energies of these outer orbitals become higher (less stabilized by nuclear K charge). n s 4 3 2 1 p d f

Shielding l l The s orbitals have greater probability density near the nucleus than p or d in the same shell and experience less shielding. Consequently, the energy ordering in a shell is Lower energy 3 p 3 d 3 s

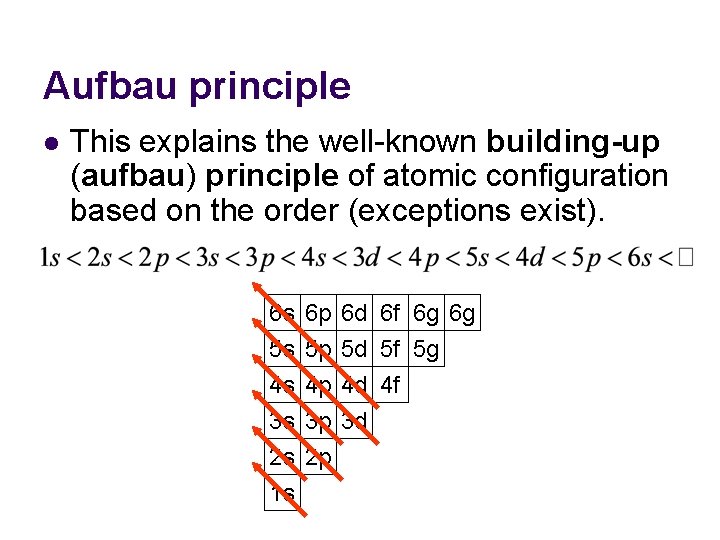
Aufbau principle l This explains the well-known building-up (aufbau) principle of atomic configuration based on the order (exceptions exist). 6 s 5 s 4 s 3 s 2 s 1 s 6 p 5 p 4 p 3 p 2 p 6 d 6 f 6 g 6 g 5 d 5 f 5 g 4 d 4 f 3 d

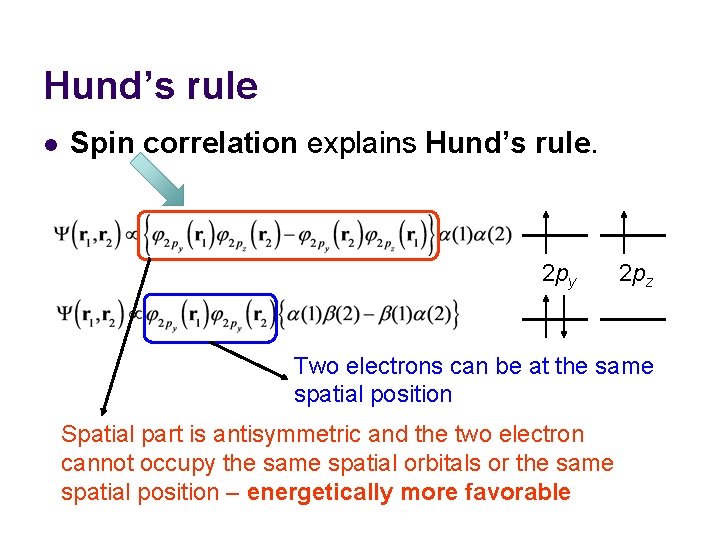
Hund’s rule l An atom in its ground state adopts a configuration with the greatest number of unpaired electrons (exceptions exist) – why? 2 px 2 py 2 s 1 s Oxygen 2 pz

Hund’s rule l Spin correlation explains Hund’s rule. 2 py 2 pz Two electrons can be at the same spatial position Spatial part is antisymmetric and the two electron cannot occupy the same spatial orbitals or the same spatial position – energetically more favorable

Summary l l l We have learned the orbital approximation, an approximate wave function of a many-electron atom that is an antisymmetric product of hydrogenic orbitals. We have learned how the (anti)symmetry of spin part affects the spatial part and hence the energies. Electron-electron Coulomb repulsion is responsible for spin correlation and shielding. Spin correlation, in turn, leads to Hund’s rule, and shielding explains the aufbau principle.
 Periodic table of elements regents
Periodic table of elements regents These vessels have thicker walls and a heavier tunica media
These vessels have thicker walls and a heavier tunica media What gas is heavier than air
What gas is heavier than air Mass and weight examples
Mass and weight examples Do heavier objects fall faster
Do heavier objects fall faster Do heavier things fall faster
Do heavier things fall faster Do heavier objects fall faster
Do heavier objects fall faster How elements heavier than iron are formed
How elements heavier than iron are formed Do heavier objects fall faster
Do heavier objects fall faster Lord kelvin flying machines
Lord kelvin flying machines Shell shock symptoms
Shell shock symptoms 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Physical and chemical properties of helium
Physical and chemical properties of helium Volume helium pour soulever 1kg
Volume helium pour soulever 1kg Heliumportal
Heliumportal Helium recovery line
Helium recovery line Bohr diagram for oxygen
Bohr diagram for oxygen Helium liquefaction temperature
Helium liquefaction temperature Helium fun facts
Helium fun facts Helium collector
Helium collector Helium nickname
Helium nickname Hitunglah laju bunyi di dalam helium
Hitunglah laju bunyi di dalam helium Valence electrons of helium
Valence electrons of helium Helium tanm
Helium tanm Balon udara berbentuk bola diisi dengan helium
Balon udara berbentuk bola diisi dengan helium