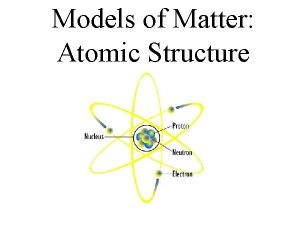
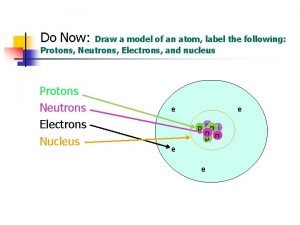
Atoms and Molecules Atoms Electrons Hydrogen Helium Neutrons













- Slides: 36

Atoms and Molecules

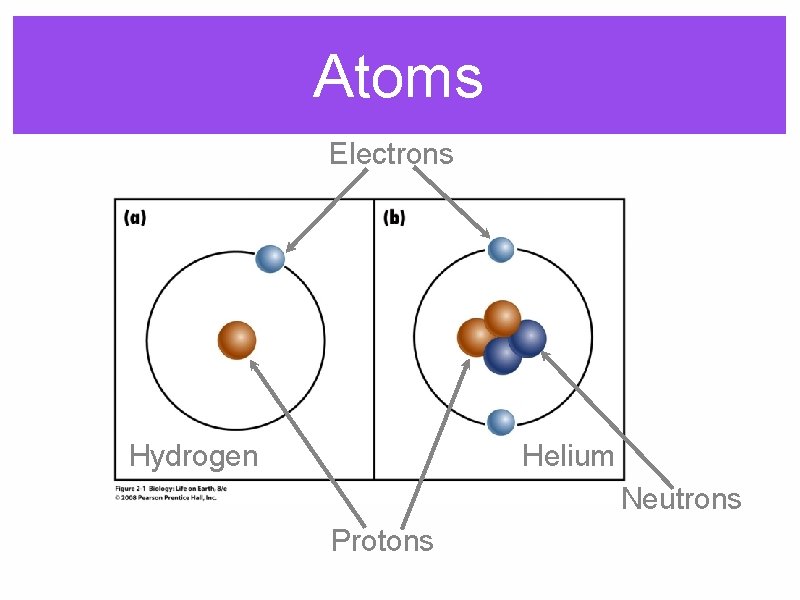
Atoms Electrons Hydrogen Helium Neutrons Protons

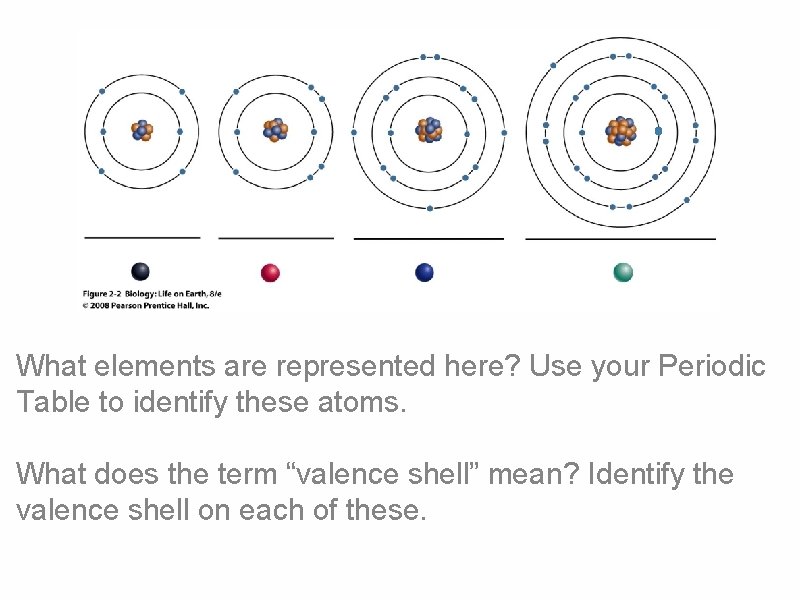
What elements are represented here? Use your Periodic Table to identify these atoms. What does the term “valence shell” mean? Identify the valence shell on each of these.

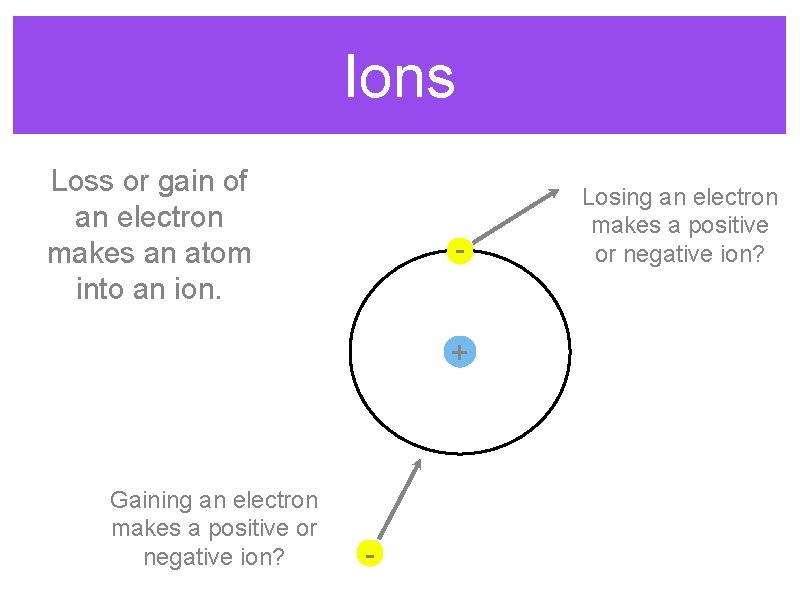
Ions Loss or gain of an electron makes an atom into an ion. + Gaining an electron makes a positive or negative ion? - Losing an electron makes a positive or negative ion?

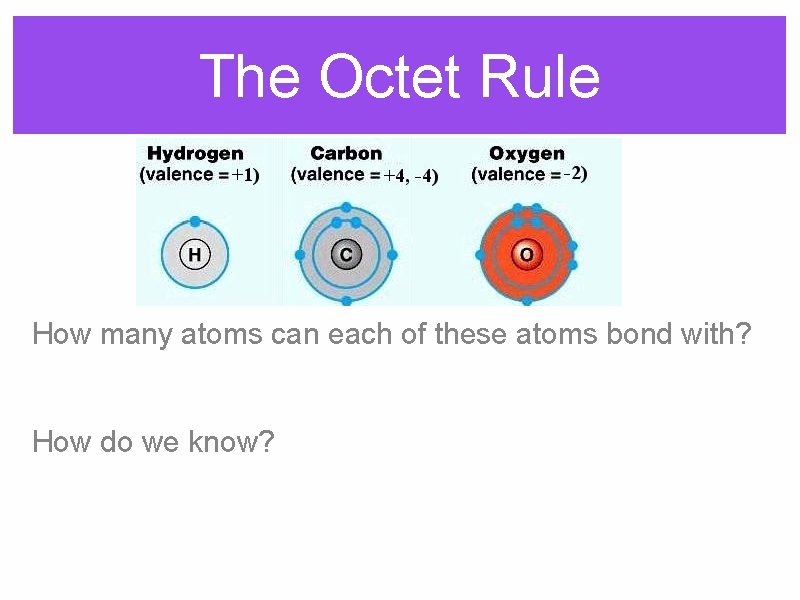
The Octet Rule • Atoms bond to fill their Valence Shell to 8 electrons

The Octet Rule How many atoms can each of these atoms bond with? How do we know?

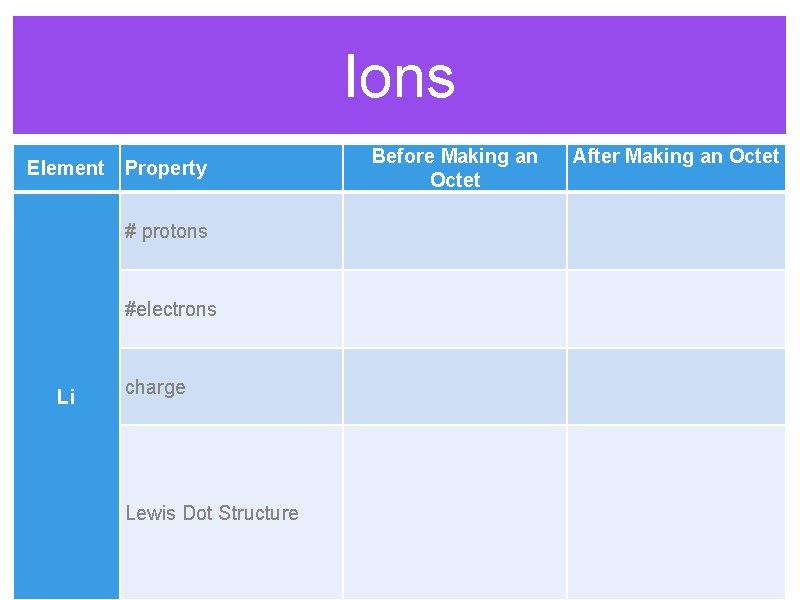
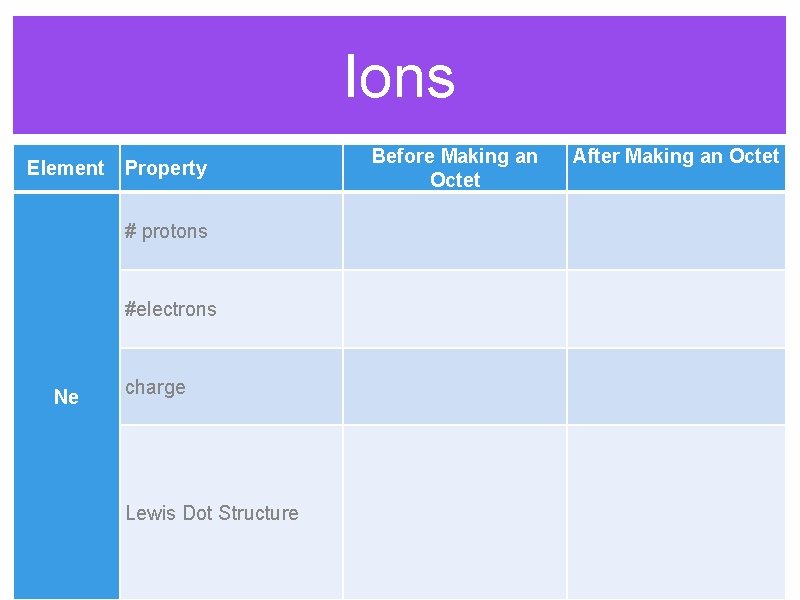
Ions Before Making an Octet Element Property After Making an Octet # protons #electrons Li charge Lewis Dot Structure

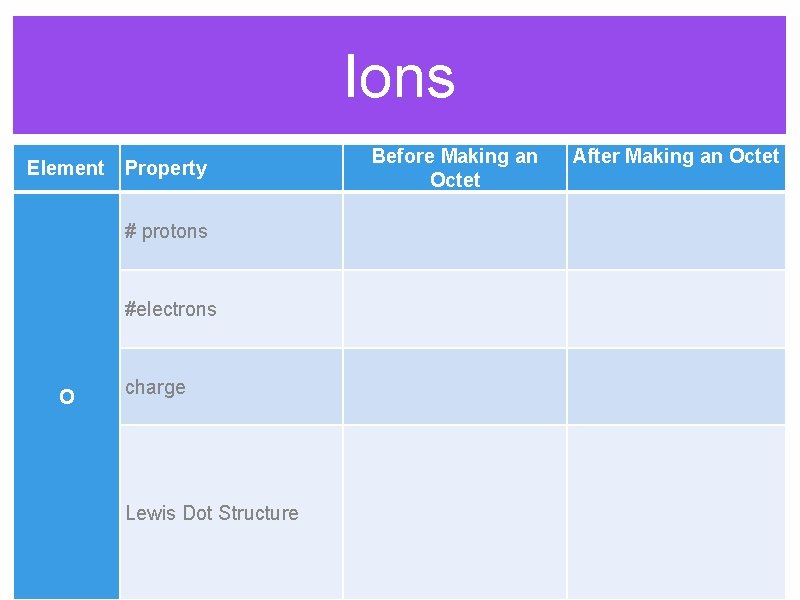
Ions Before Making an Octet Element Property After Making an Octet # protons #electrons O charge Lewis Dot Structure

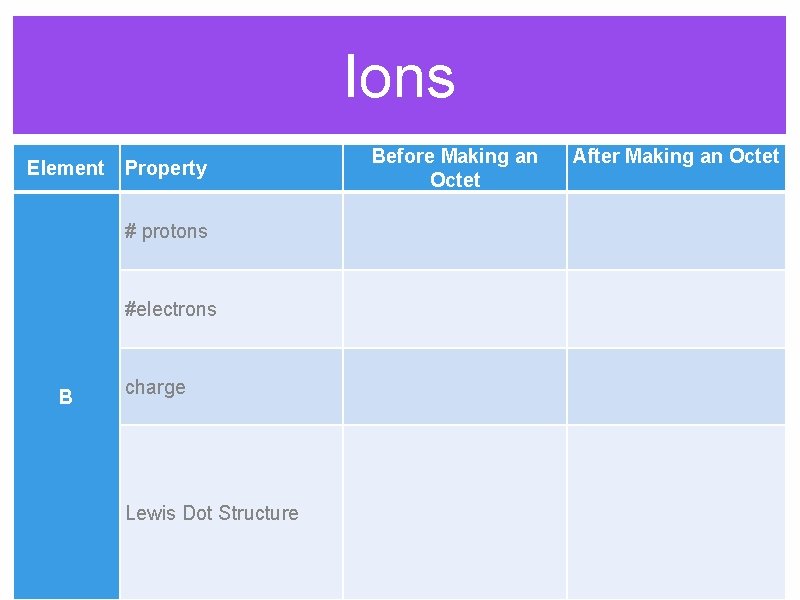
Ions Before Making an Octet Element Property After Making an Octet # protons #electrons B charge Lewis Dot Structure

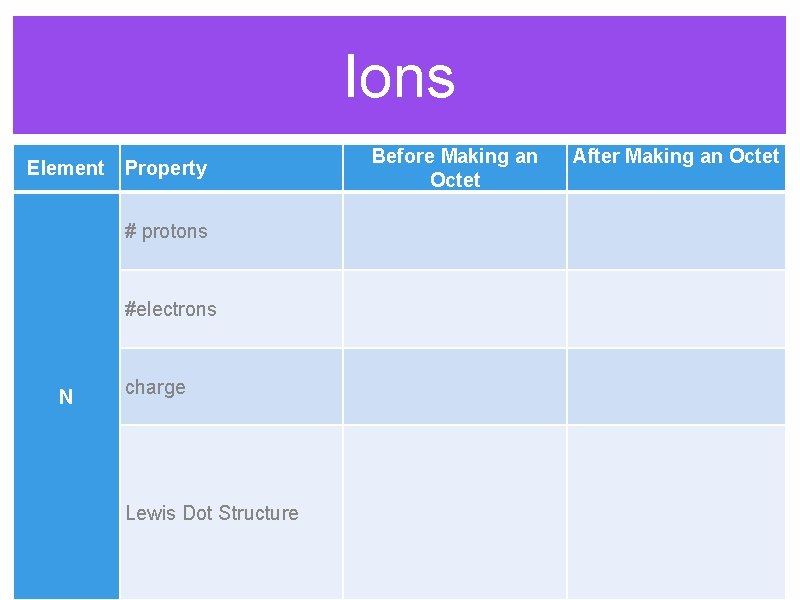
Ions Before Making an Octet Element Property After Making an Octet # protons #electrons N charge Lewis Dot Structure

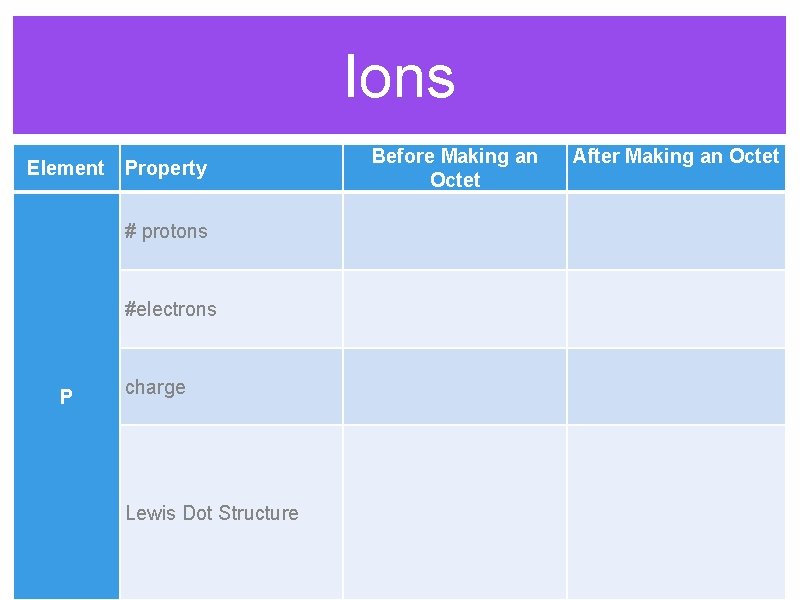
Ions Before Making an Octet Element Property After Making an Octet # protons #electrons P charge Lewis Dot Structure

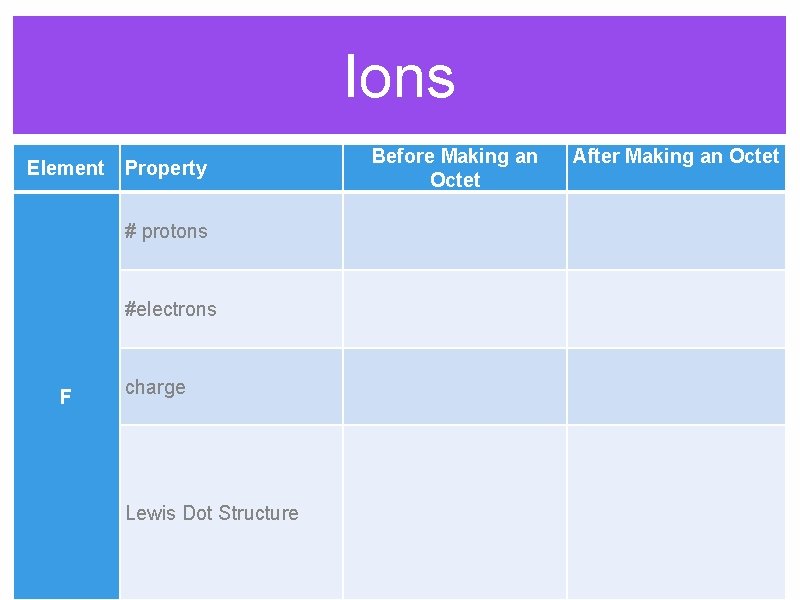
Ions Before Making an Octet Element Property After Making an Octet # protons #electrons F charge Lewis Dot Structure

Ions Before Making an Octet Element Property After Making an Octet # protons #electrons Ne charge Lewis Dot Structure

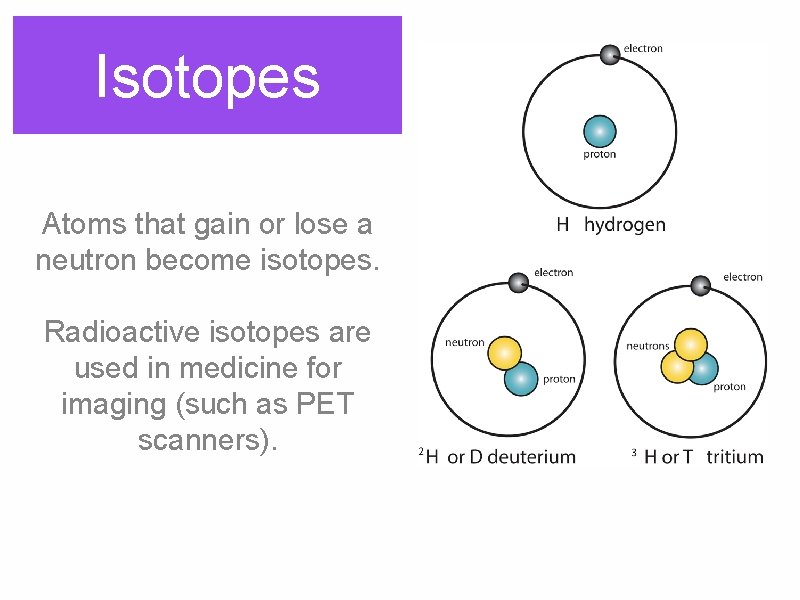
Isotopes Atoms that gain or lose a neutron become isotopes. Radioactive isotopes are used in medicine for imaging (such as PET scanners).

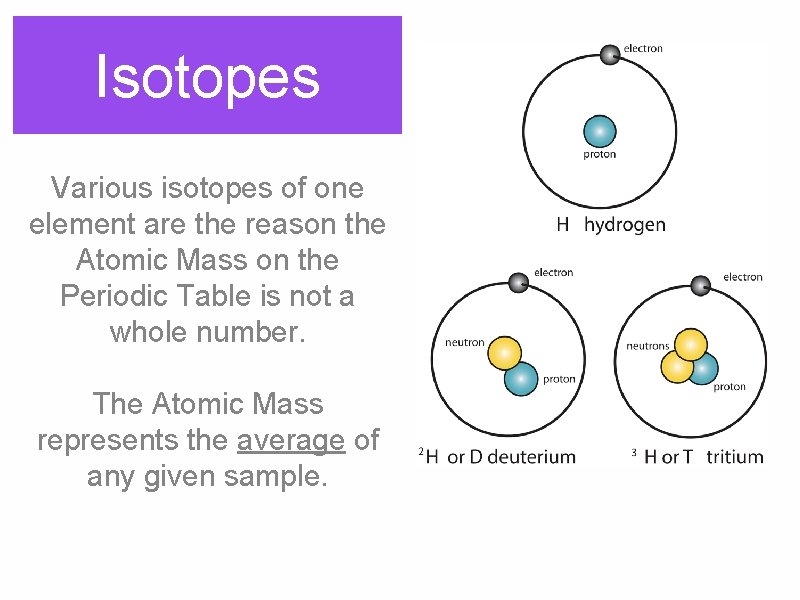
Isotopes Various isotopes of one element are the reason the Atomic Mass on the Periodic Table is not a whole number. The Atomic Mass represents the average of any given sample.

Atoms bond together • Molecules are made up of atoms bonded together. • The structure of an individual atom determines: • Whether the atom can form bonds. • How many other atoms it can bond to.

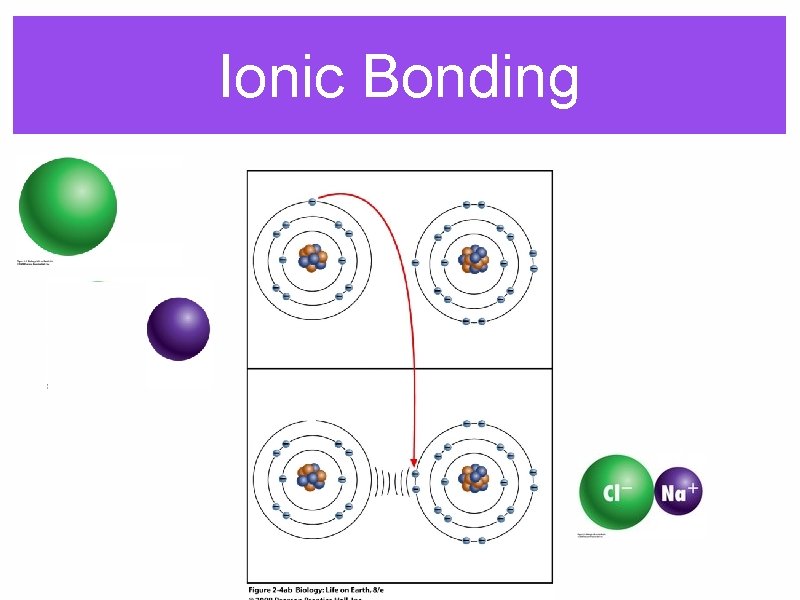
Ionic Bonding

Ionic substances tend to form crystaline lattices rather than distinct molecules.

Ionic Bonding For each of the following ionic bonds: • Write the symbols for each element. • Draw a Lewis Dot structure for the valence shell of each element. • Draw an arrow (or more if needed) to show the transfer of electrons to the new element. • Write the resulting chemical formula.

Ionic Bonding Sodium + Chlorine

Ionic Bonding Magnesium + Iodine

Ionic Bonding Sodium + Oxygen

Ionic Bonding Calcium + Chlorine

Ionic Bonding Aluminum + Chlorine

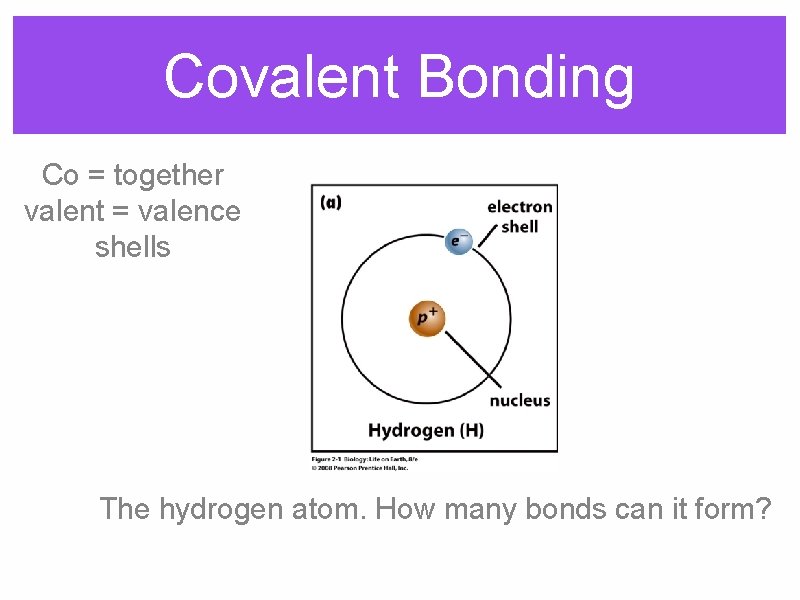
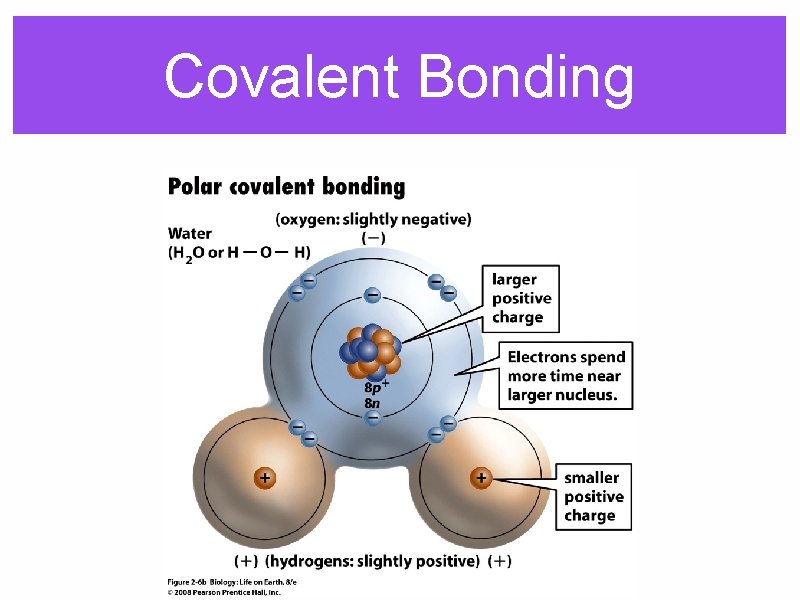
Covalent Bonding Co = together valent = valence shells The hydrogen atom. How many bonds can it form?

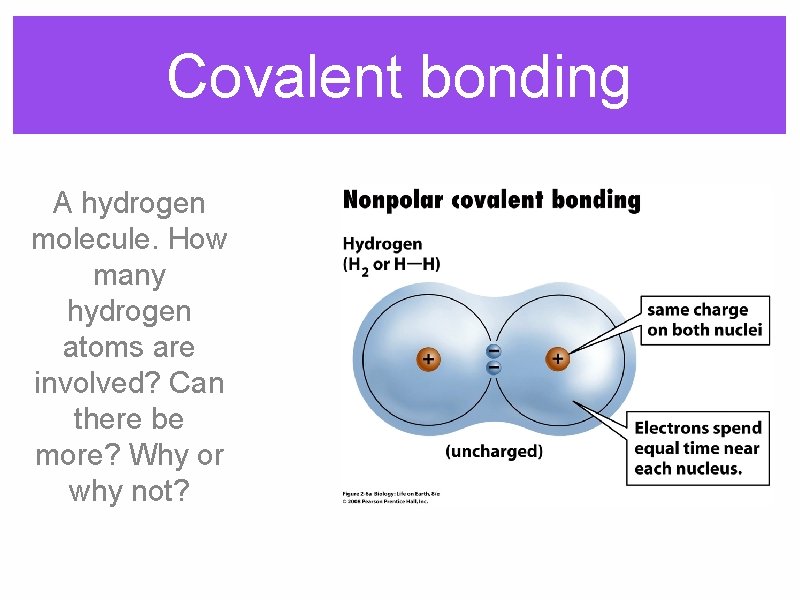
Covalent bonding A hydrogen molecule. How many hydrogen atoms are involved? Can there be more? Why or why not?

Covalent Bonding

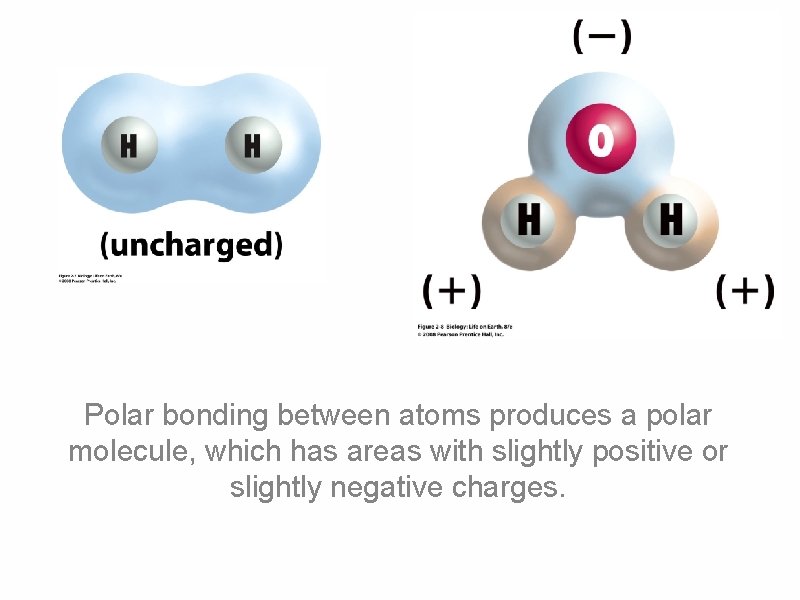
Polar bonding between atoms produces a polar molecule, which has areas with slightly positive or slightly negative charges.

Covalent Bonding For each of the following covalent bonds: • Write the symbols for each element. • Draw a Lewis Dot structure for the valence shell of each element. • Rearrange the electrons to pair up electrons from each atom. • Draw circles to show the sharing of electrons between each pair of atoms • Draw the bond structure using chemical symbols and lines. Use one line for each pair of electrons that is shared. • Write the chemical formula for each molecule.

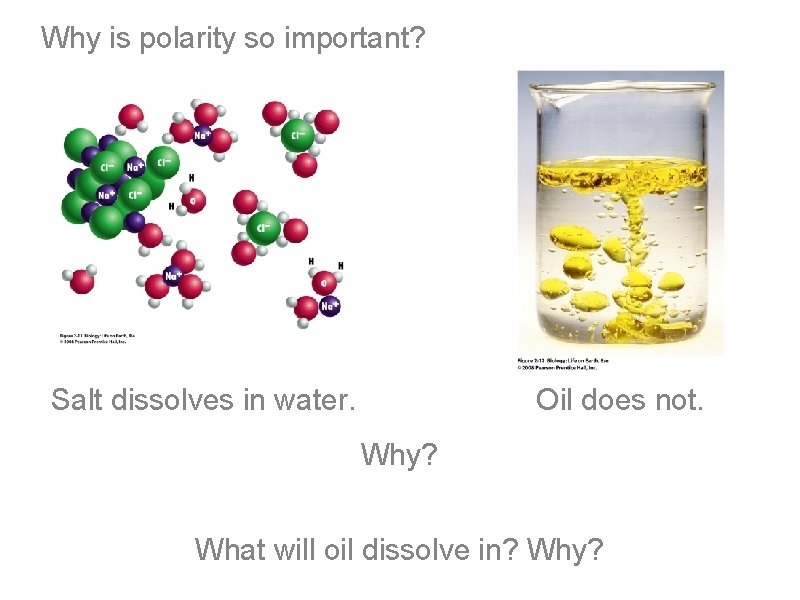
Why is polarity so important? Salt dissolves in water. Oil does not. Why? What will oil dissolve in? Why?

Covalent Bonding Hydrogen + Hydrogen

Covalent Bonding Chlorine + Chlorine

Covalent Bonding Hydrogen + Chlorine

Covalent Bonding Hydrogen + Oxygen

Covalent Bonding Nitrogen + Hydrogen

Covalent Bonding Carbon + Hydrogen
 Number of neutrons helium
Number of neutrons helium Sulfur number of neutrons protons and electrons
Sulfur number of neutrons protons and electrons How many neutrons are in gold
How many neutrons are in gold Neutrons in hydrogen
Neutrons in hydrogen Valence electrons of helium
Valence electrons of helium How many orbitals are there in the third shell of ytterbium
How many orbitals are there in the third shell of ytterbium How electrons are arranged in an atom
How electrons are arranged in an atom What is an atom inventory
What is an atom inventory How to find protons and electrons
How to find protons and electrons Lithium protons neutrons electrons
Lithium protons neutrons electrons Nonmetallic, period 3, atomic mass 32
Nonmetallic, period 3, atomic mass 32 Chromium 63 protons neutrons electrons
Chromium 63 protons neutrons electrons 24mg12 2+
24mg12 2+ Democritus atomic theory
Democritus atomic theory 39k+ protons neutrons electrons
39k+ protons neutrons electrons 39k+ protons neutrons electrons
39k+ protons neutrons electrons U 238 protons neutrons electrons
U 238 protons neutrons electrons Nitrogen
Nitrogen Molecules containing hydrogen
Molecules containing hydrogen Organic molecules vs inorganic molecules
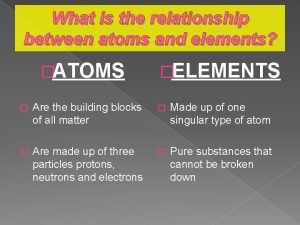
Organic molecules vs inorganic molecules What is the relationship between atoms and elements
What is the relationship between atoms and elements Study jams mixtures
Study jams mixtures How can you count atoms and molecules
How can you count atoms and molecules Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules Collision theory states that
Collision theory states that Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Mri hydrogen atoms
Mri hydrogen atoms Bingabr
Bingabr