Formation of Heavier Elements AN EXPLANATION OF HOW


- Slides: 7

Formation of Heavier Elements AN EXPLANATION OF HOW ELEMENTS HEAVIER THAN FE ARE PRODUCED IN SUPERNOVAE

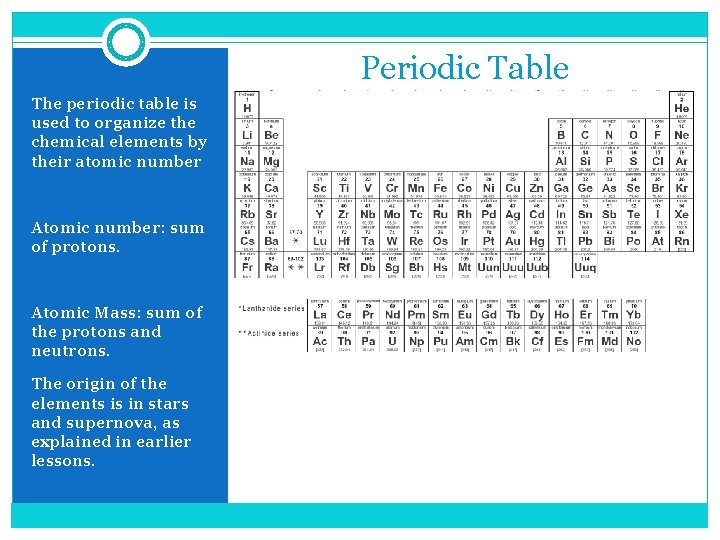
Periodic Table The periodic table is used to organize the chemical elements by their atomic number Atomic number: sum of protons. Atomic Mass: sum of the protons and neutrons. The origin of the elements is in stars and supernova, as explained in earlier lessons.

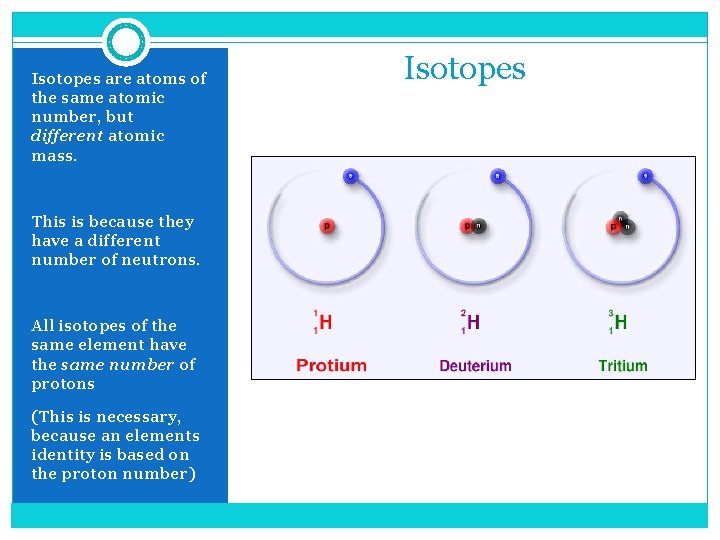
Isotopes are atoms of the same atomic number, but different atomic mass. This is because they have a different number of neutrons. All isotopes of the same element have the same number of protons (This is necessary, because an elements identity is based on the proton number) Isotopes

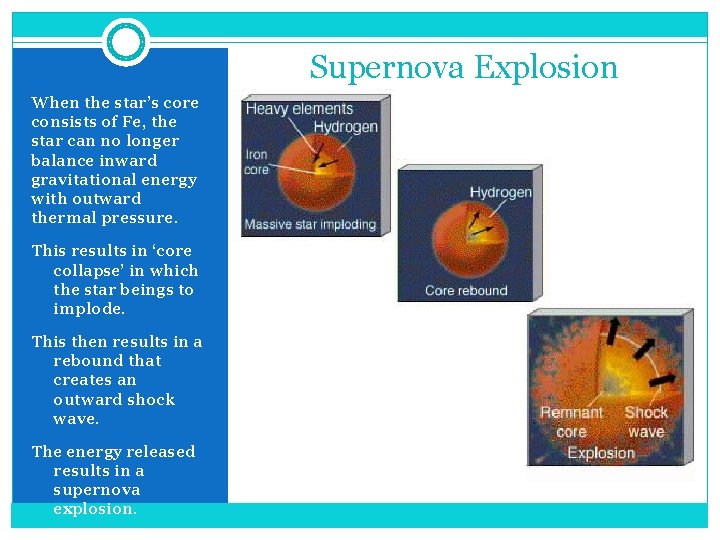
Supernova Explosion When the star’s core consists of Fe, the star can no longer balance inward gravitational energy with outward thermal pressure. This results in ‘core collapse’ in which the star beings to implode. This then results in a rebound that creates an outward shock wave. The energy released results in a supernova explosion.

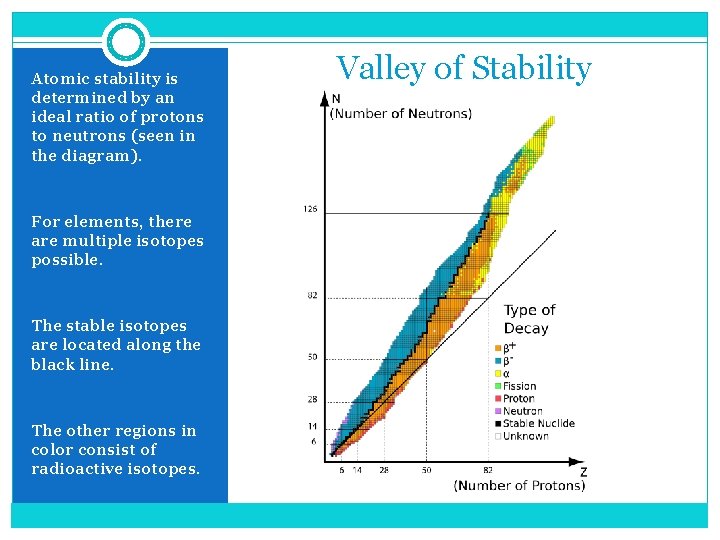
Atomic stability is determined by an ideal ratio of protons to neutrons (seen in the diagram). For elements, there are multiple isotopes possible. The stable isotopes are located along the black line. The other regions in color consist of radioactive isotopes. Valley of Stability

This image is titled “The Pillars of Creation” and was taken by the Hubble Telescope in 1995. It is an image of a gas and dust in the Eagle Nebula. It is an iconic image and is a reminder that the beginnings of the Solar System and life on Earth come from these primitive clouds of dust and gas. Made of Stars

Summary Elements heavier than iron (Fe) are formed during a Type II supernova explosion The elements on the periodic table are organized according to their atomic number (Z, which is equivalent to the number of protons). Isotopes are different forms of the same element, with different atomic masses. This is because isotopes of an element have a different number of neutrons (but the same number of protons). The stability of a nucleus is determined by the ratio of neutrons to protons. Unstable nuclides are subject to radioactive decay