Nucleus Protons and Neutrons Subatomic Particles Protons mass
Nucleus – Protons and Neutrons
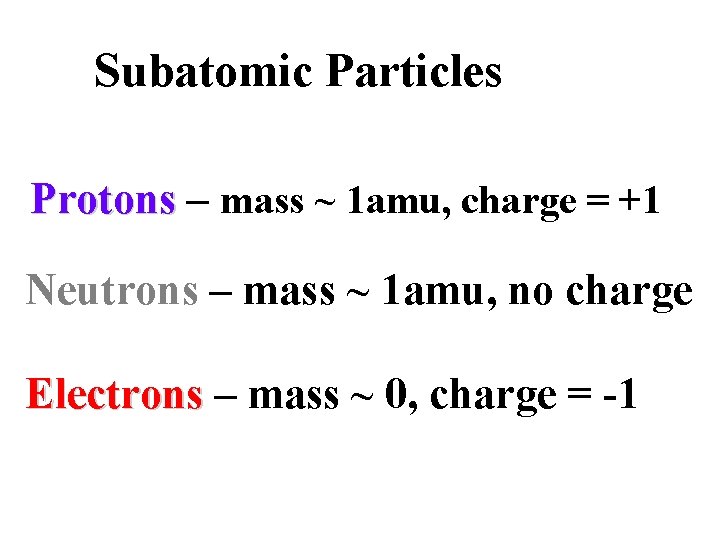
Subatomic Particles Protons – mass ~ 1 amu, charge = +1 Neutrons – mass ~ 1 amu, no charge Electrons – mass ~ 0, charge = -1
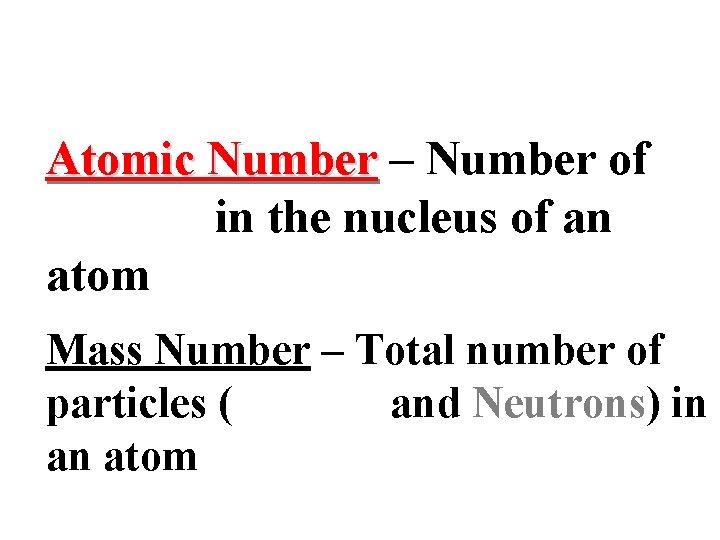
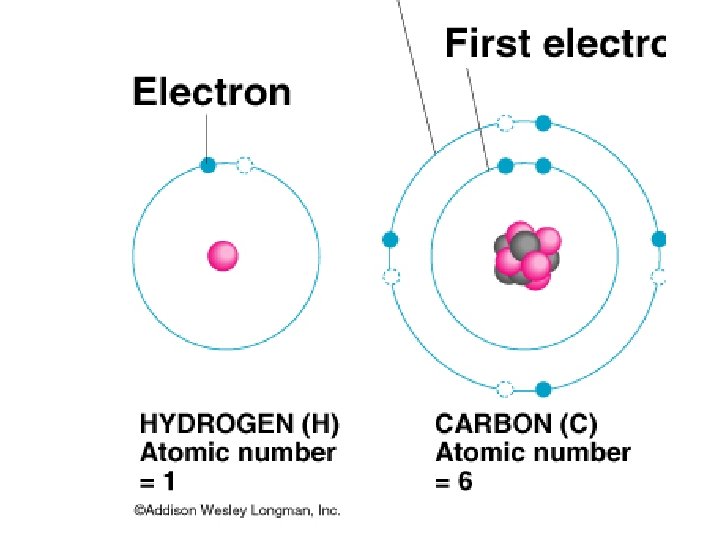
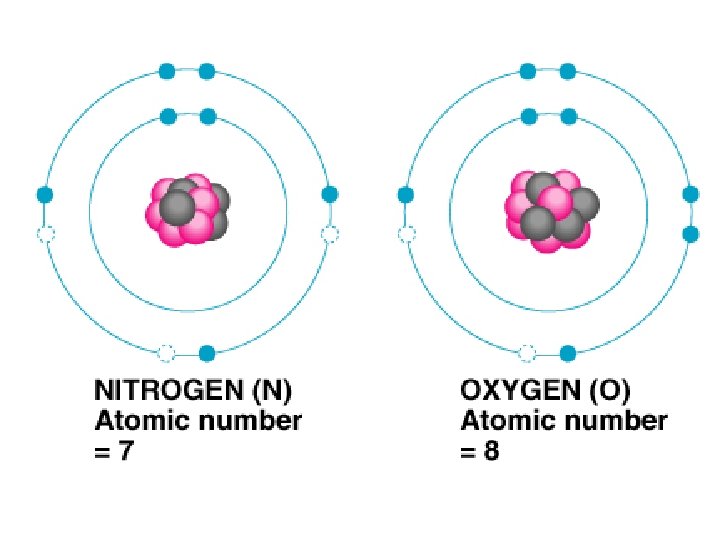
Atomic Number – Number of protons in the nucleus of an atom Mass Number – Total number of particles (Protons and Neutrons) in an atom
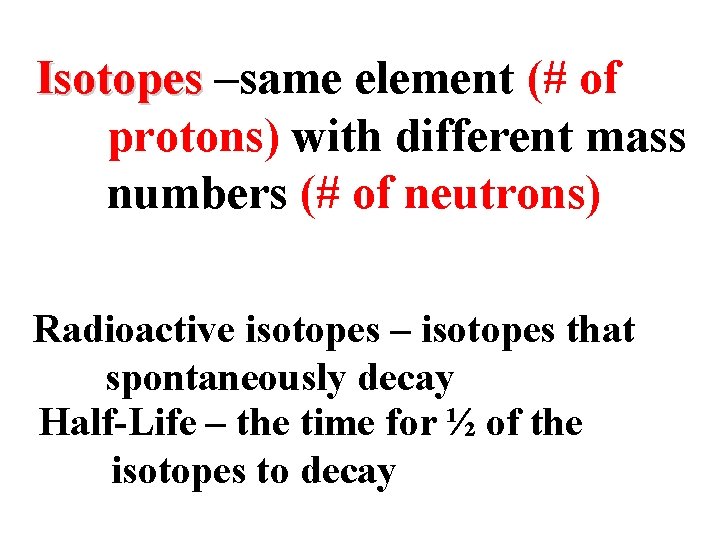
Isotopes –same element (# of protons) with different mass numbers (# of neutrons) Radioactive isotopes – isotopes that spontaneously decay Half-Life – the time for ½ of the isotopes to decay
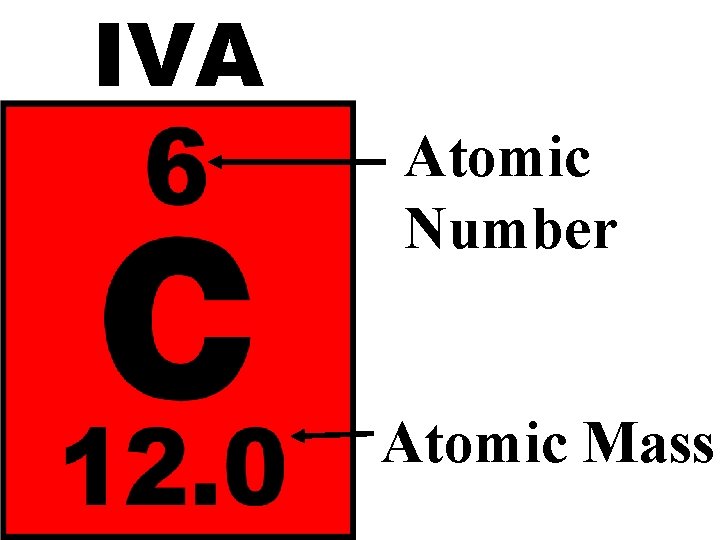
IVA Atomic Number Atomic Mass
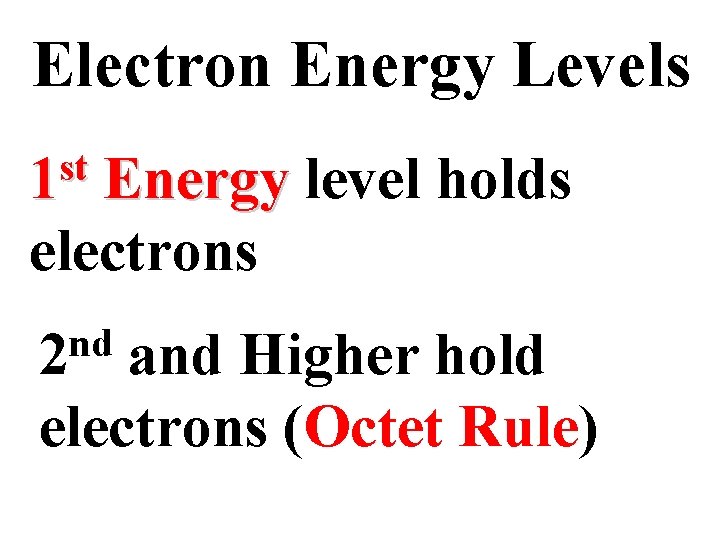
Electron Energy Levels st 1 Energy level holds 2 electrons nd 2 and Higher hold 8 electrons (Octet Rule)
http: //sci 2 k. net/periodictable. html 1222222345678
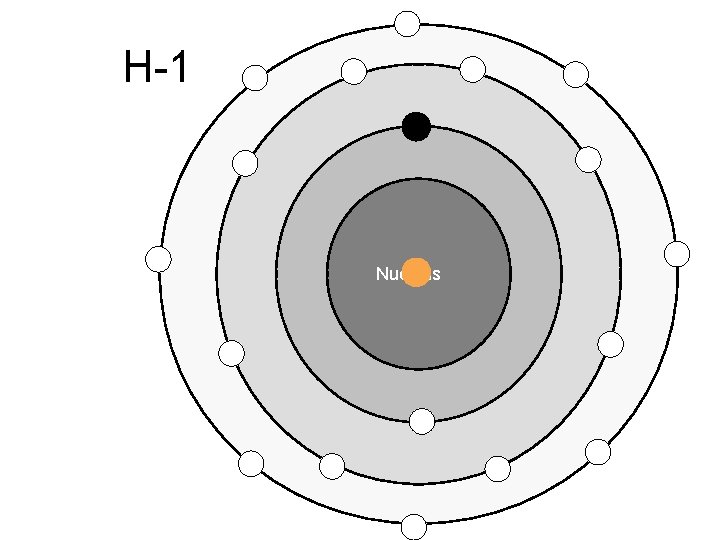
H-1 Nucleus
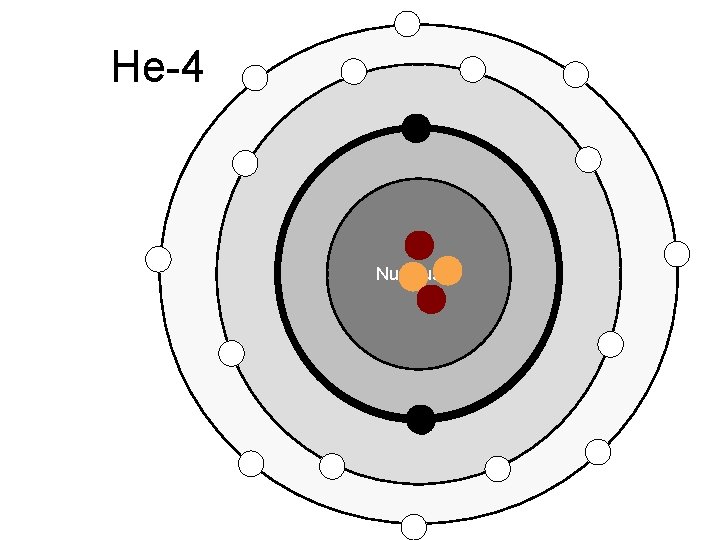
He-4 Nucleus
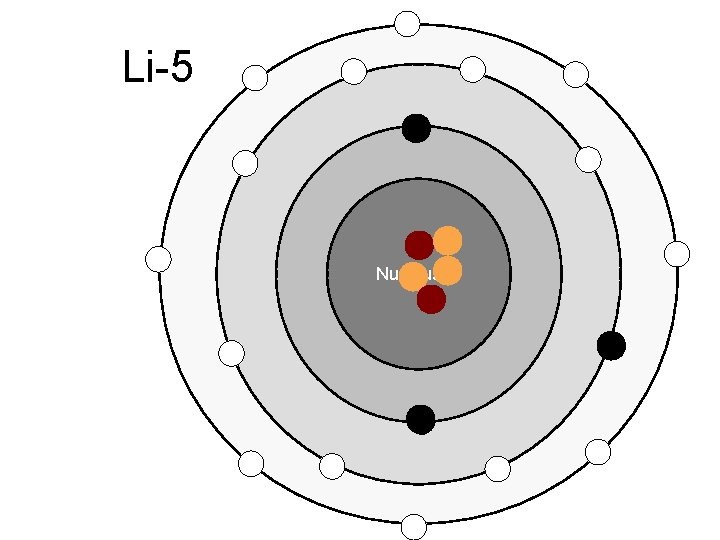
Li-5 Nucleus
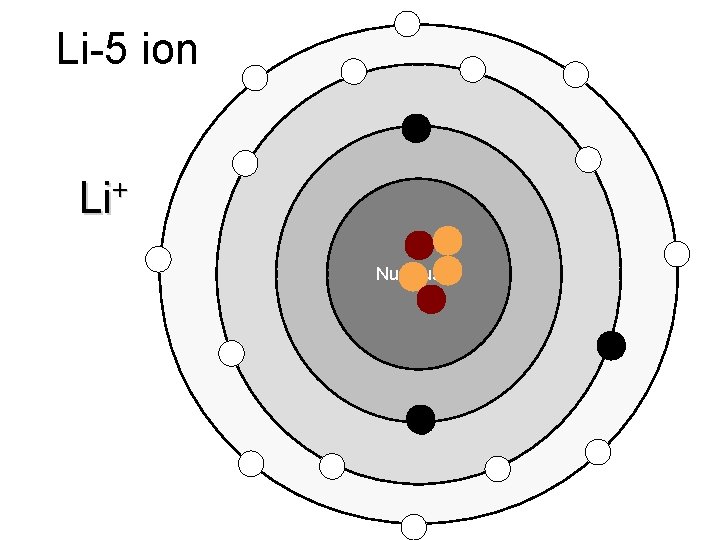
Li-5 ion + Li Nucleus
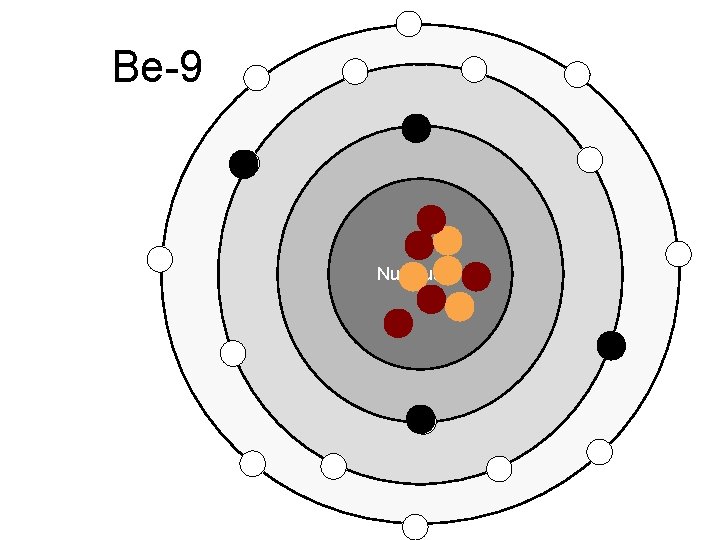
Be-9 Nucleus
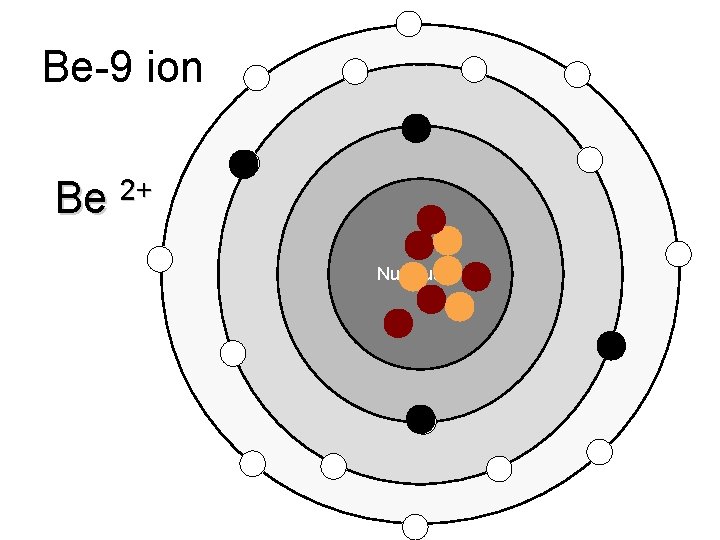
Be-9 ion Be 2+ Nucleus
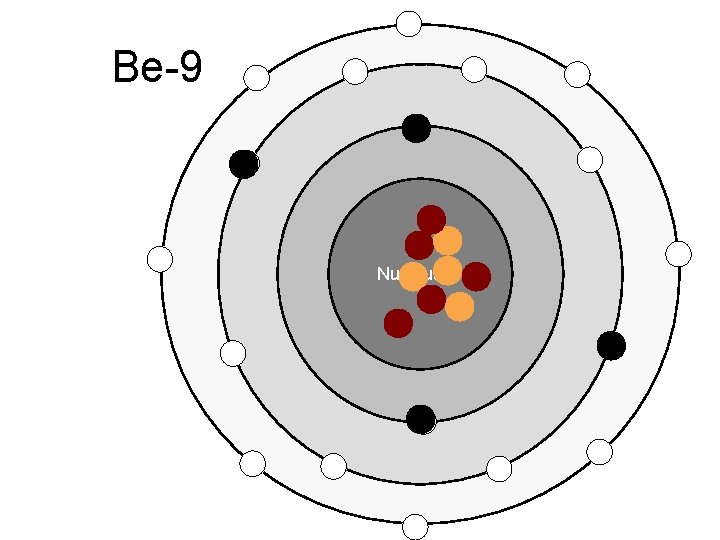
Be-9 Nucleus
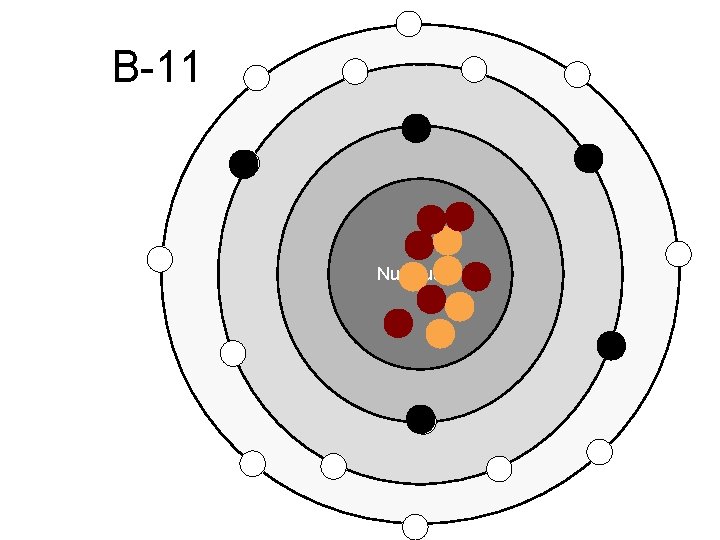
B-11 Nucleus
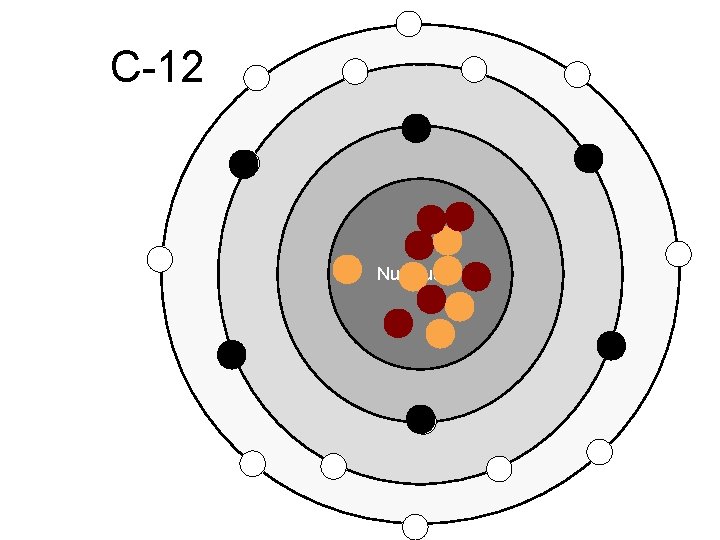
C-12 Nucleus
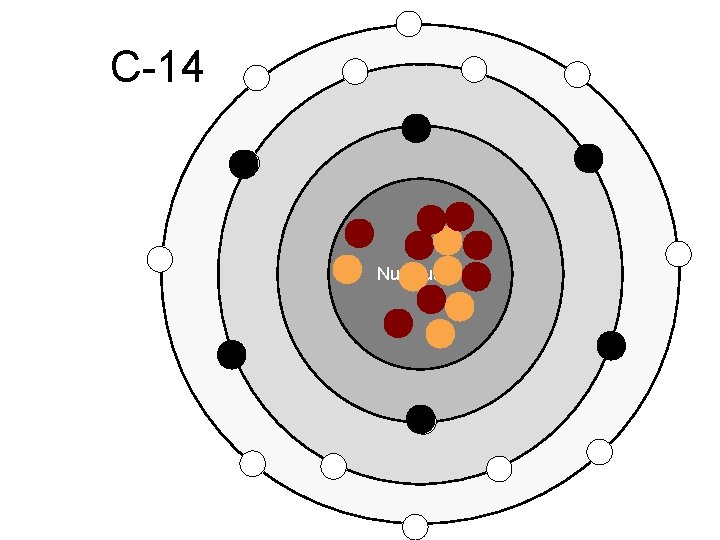
C-14 Nucleus
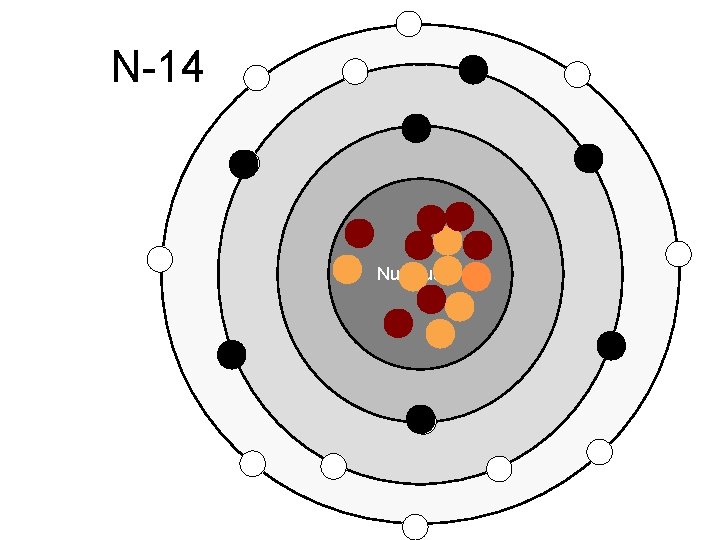
N-14 Nucleus
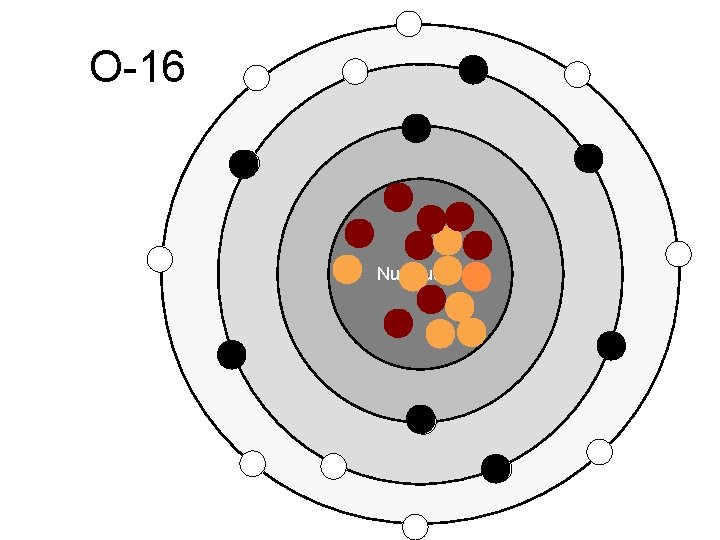
O-16 Nucleus
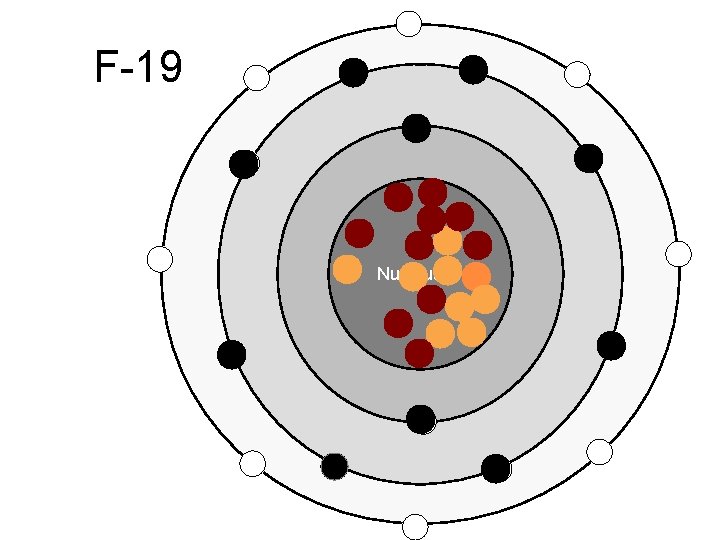
F-19 Nucleus
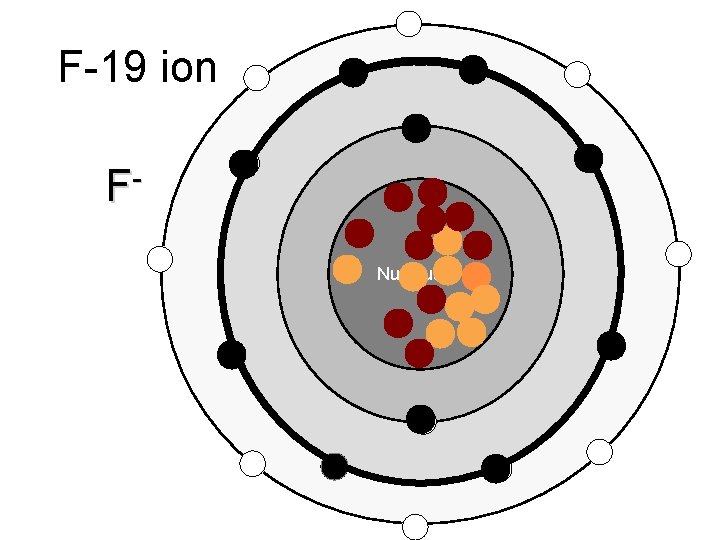
F-19 ion F Nucleus
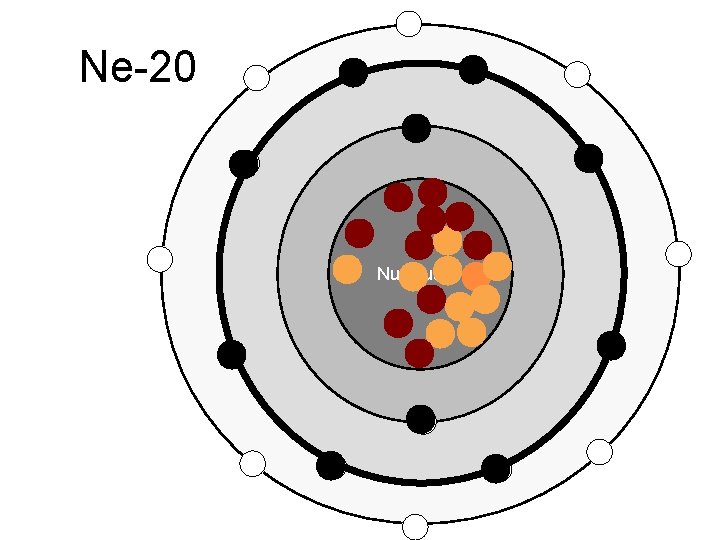
Ne-20 Nucleus
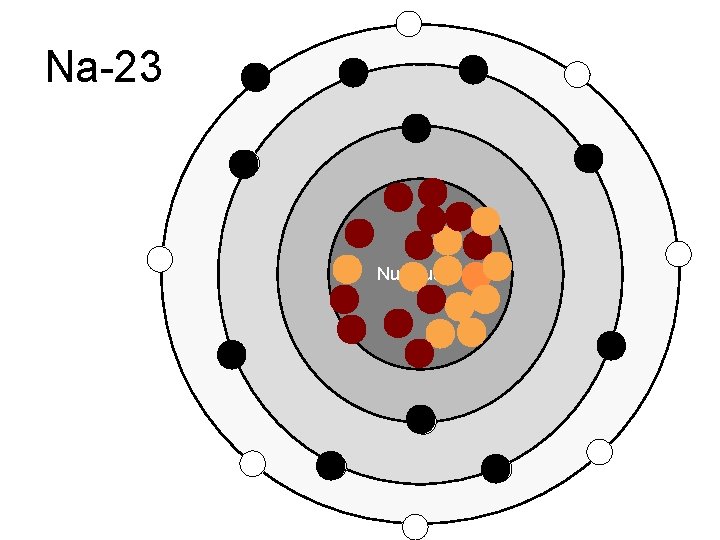
Na-23 Nucleus
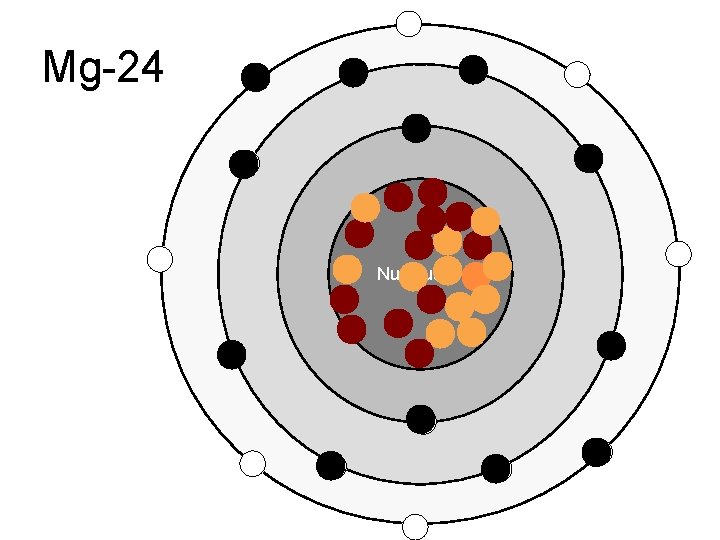
Mg-24 Nucleus
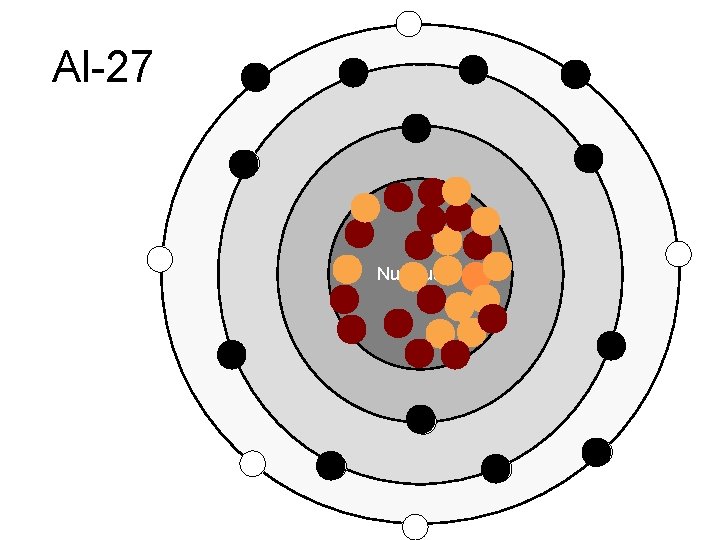
Al-27 Nucleus
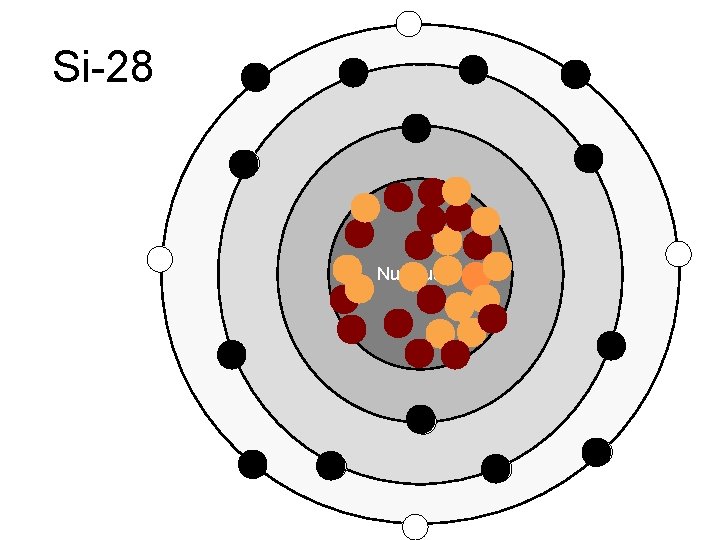
Si-28 Nucleus

Quiz 1. An atom of Oxygen-17 would have ___ protons. 2. An atom of Sulfur-33 would have ___ electrons. 3. An atom of Helium-5 would have ___ neutrons.

Quiz 4. An atom is discovered with 12 electrons, 12 protons and 13 neutrons. What is it?

Quiz 1. An atom of Oxygen-17 would have ___ protons. 8 protons 2. An atom of Sulfur-33 would have ___ electrons. 16 electrons 3. An atom of Helium-5 would have ___ neutrons. 3 neutrons

Quiz 4. An atom is discovered with 12 electrons, 12 protons and 13 neutrons. What is it? Magnesium-25
Metals • Properties of Metals – Shiny – luster – Conductors (electricity and heat) – Malleable Solids (except Hg) • Metals – tend to lose electrons when forming ions • Usually 3 or less e-s in outer level

Metals • Properties of Metals – Shiny – luster – Conductors (electricity and heat) – Malleable Solids (except Hg) • Metals – tend to lose electrons when forming ions • Usually 3 or less e-s in outer level
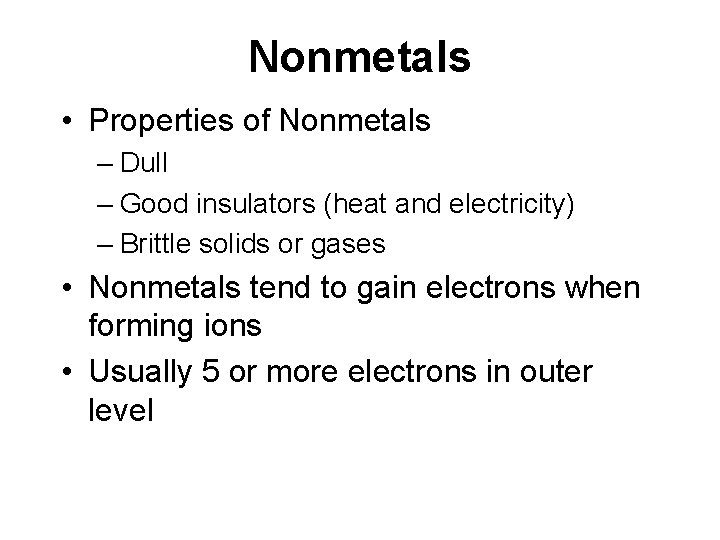
Nonmetals • Properties of Nonmetals – Dull – Good insulators (heat and electricity) – Brittle solids or gases • Nonmetals tend to gain electrons when forming ions • Usually 5 or more electrons in outer level
Nonmetals • Properties of Nonmetals – Dull – Good insulators (heat and electricity) – Brittle solids or gases • Nonmetals tend to gain electrons when forming ions • Usually 5 or more electrons in outer level
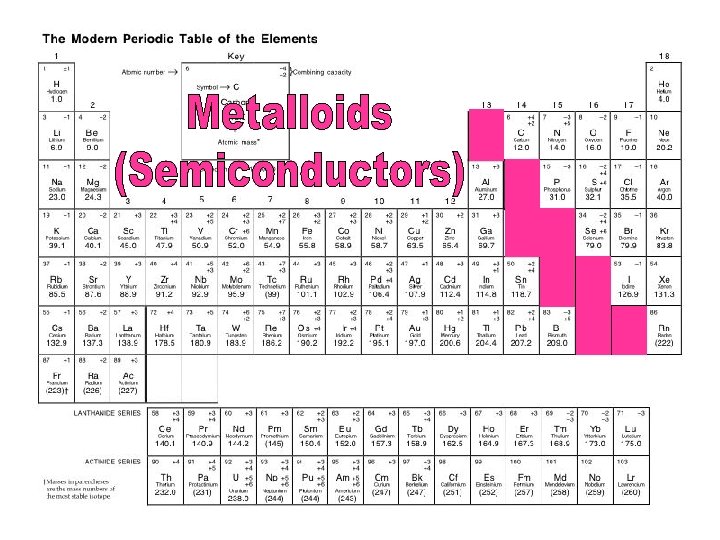
Metalloids (Semiconductors) • Metalloids have some properties of both metals and nonmetals

Pursuit of Full or Empty Energy Levels Atoms are more stable when their outer E level is full or empty To achieve this they will Steal, Give up, or Share electrons
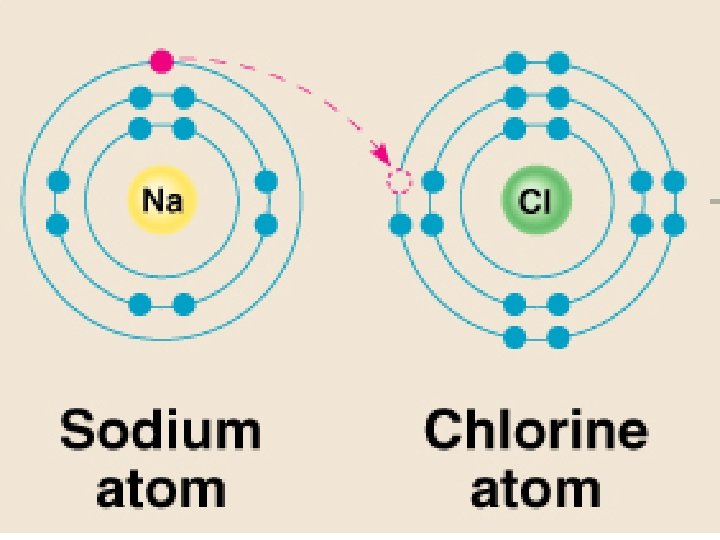
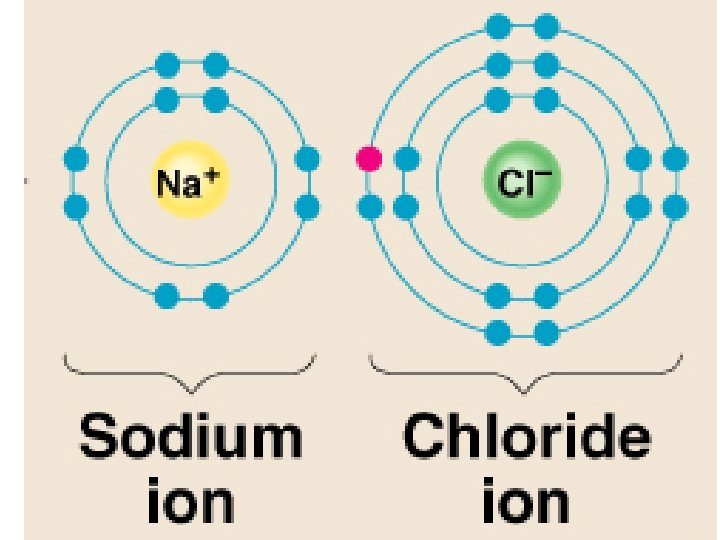

Ions – atoms that have gained or lost electrons when forming ionic bonds Cations – positive ions, have Ca+ions lost an electron (metals) Anions – negative ions, have gained electrons (nonmetals)
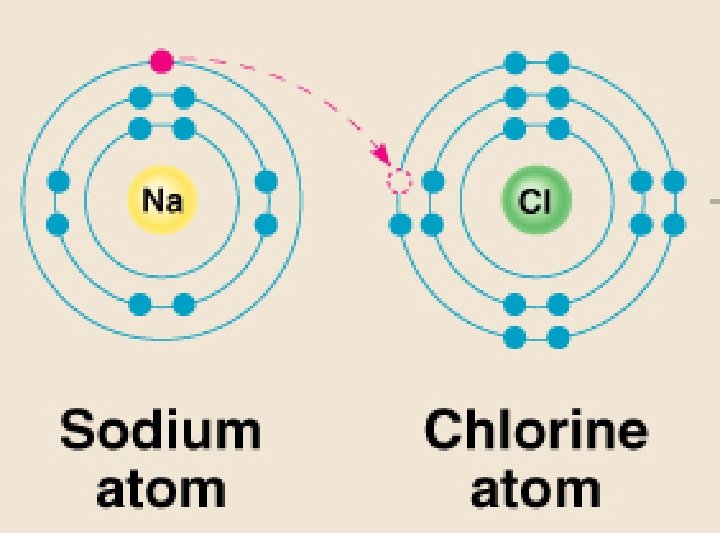
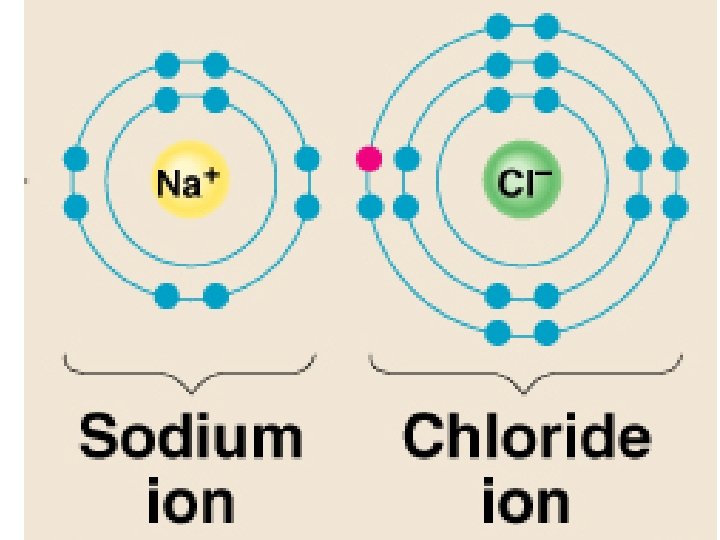

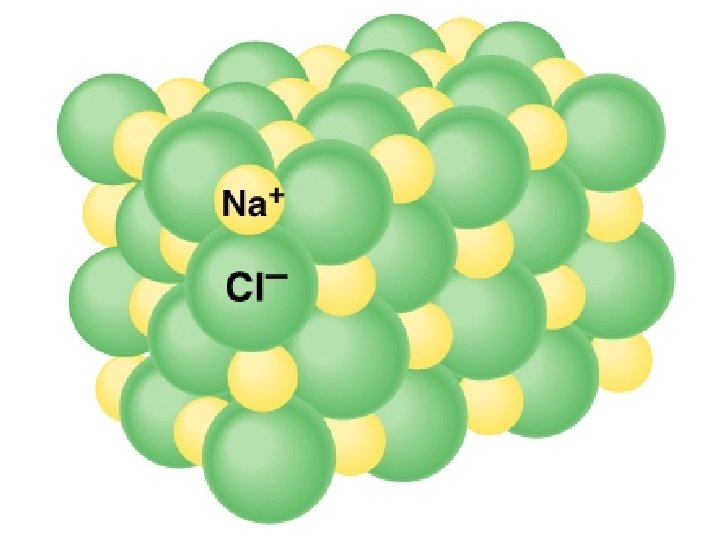
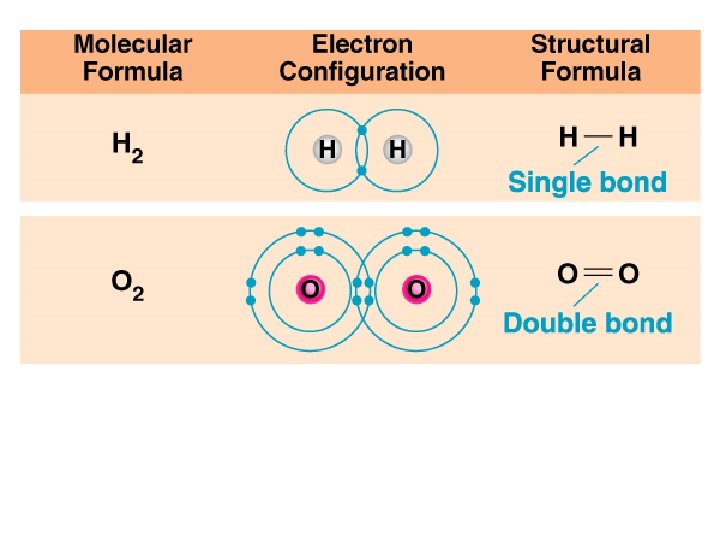
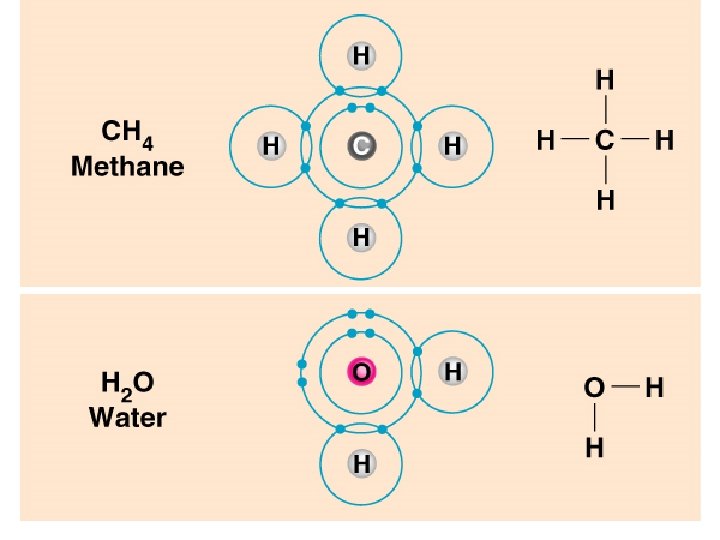
Ionic Bond – attraction between oppositely charged ions Covalent Bond – atoms share electrons to fill outer E level
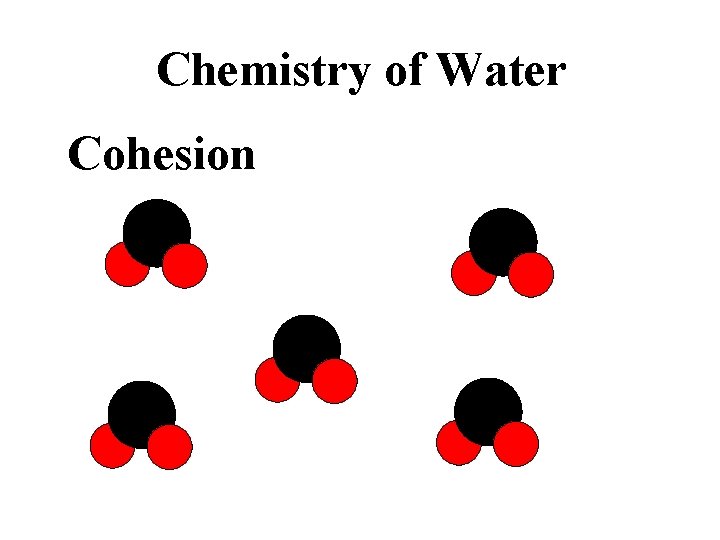
Chemistry of Water Cohesion sticks to other water molecules
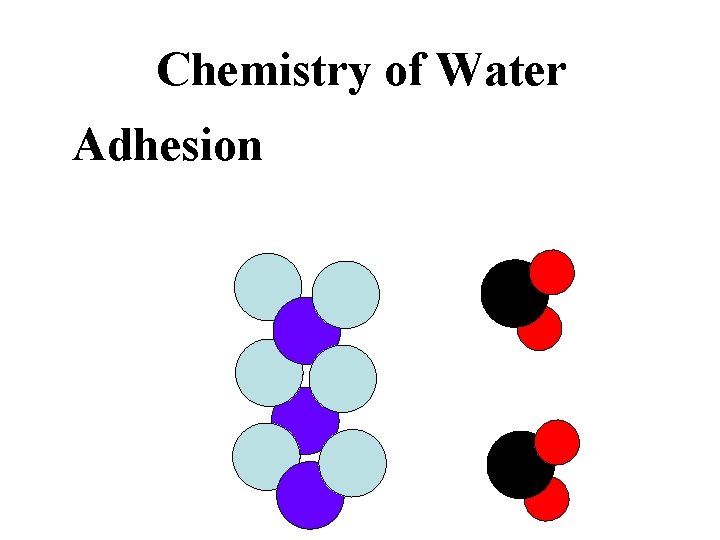
Chemistry of Water Adhesion sticks to other polar molecules


http: //www. everythingabout. net/articles/biology/animals/arthropods/insects/ bugs/water_strider. html

Chemistry of Water Specific Heat - holds large amounts of heat, stabilizes temperature Universal Solvent - dissolves most ionic compounds (salts)

Chemistry of Water Special Thermal Expansion Water increases in volume when it freezes Making ice less dense than liquid water
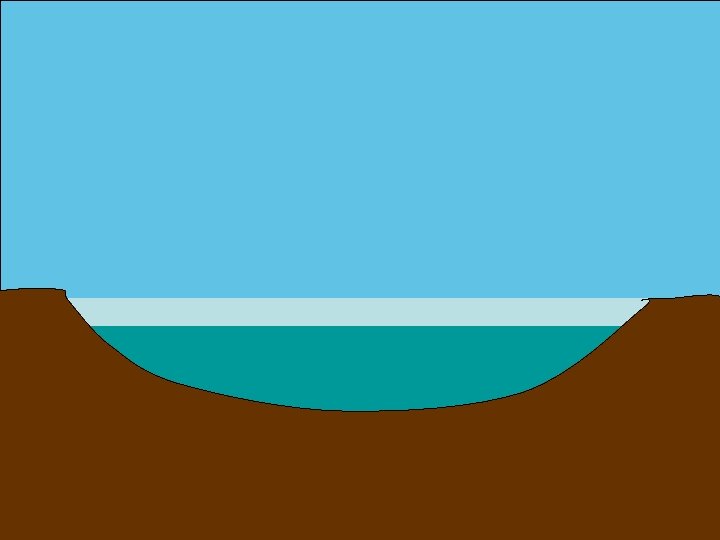


http: //www. everythingabout. net/articles/biology/animals/arthropods/insects/ bugs/water_strider. html


Water is the “Universal Solvent” Solution – a uniform mixture Solvent - dissolves the other substance Solute – substance that is dissolved
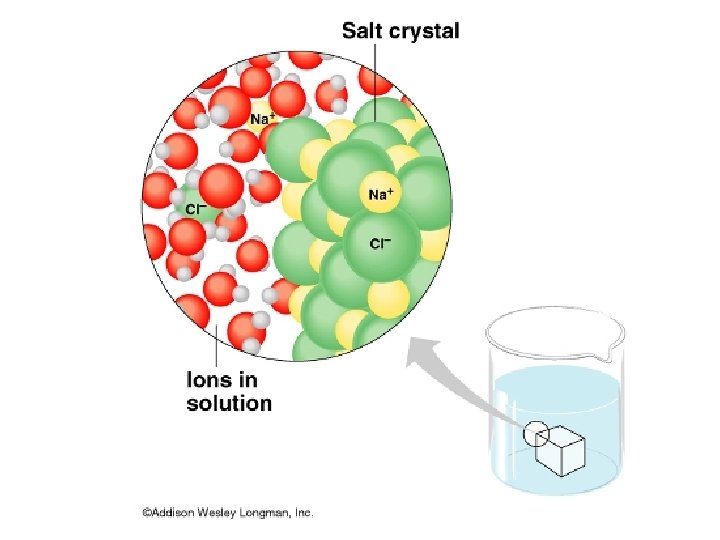

Hydrophilic water loving Ionic or polar substances (Salts) Hydrophobic water fearing nonpolar substances (oils)
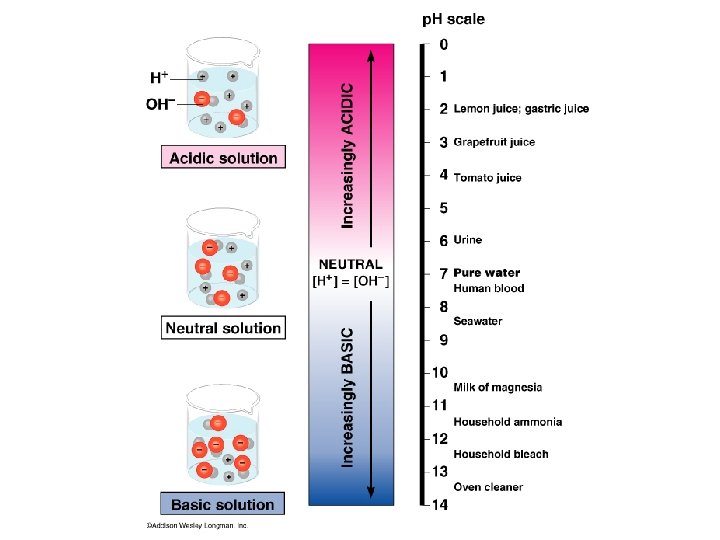

Acids - p. H less than 7 + more H than OH ions Bases - p. H greater than 7 more OH- than H+ ions Neutral – p. H of 7 equal H+ and OH ions Buffer – controls p. H by + donating or accepting H

p. H – is based on powers of 10 p. H # is actually 10 -# p. H 7 is actually 10 -7 = 0. 0000001 p. H 1 is actually -1 = 10 0. 1 -12 = 10 p. H 12 is actually 0. 0000001


Chemical Reactions
Chemical Reactions Change Substances • Yeast + sugar => carbon dioxide and ethanol • Chemical Reactions – occur when substances undergo chemical changes to form new substances • Atoms are rearranged to form new compounds or molecules
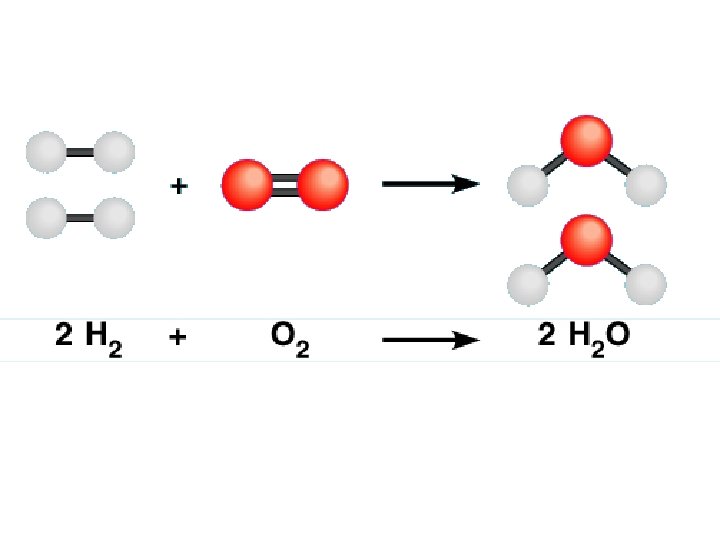
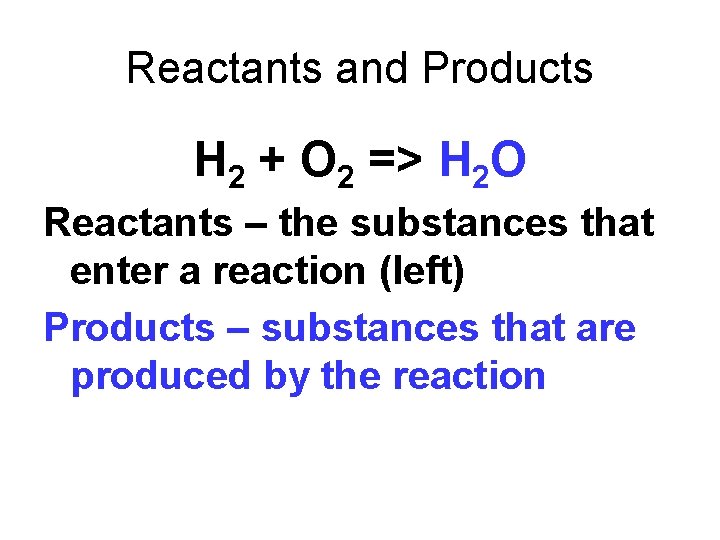
Reactants and Products H 2 + O 2 => H 2 O Reactants – the substances that enter a reaction (left) Products – substances that are produced by the reaction (right)
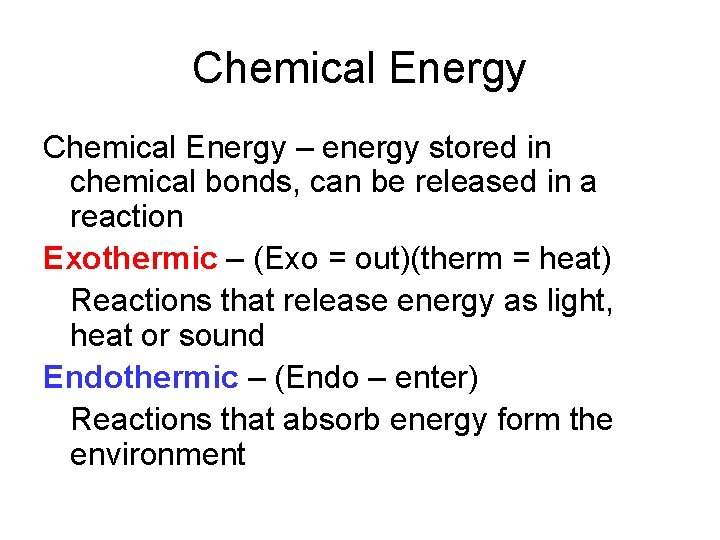
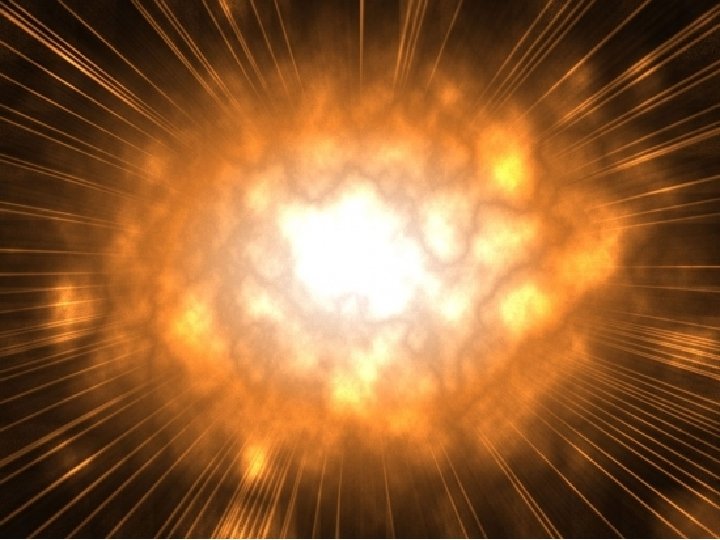
Chemical Energy – energy stored in chemical bonds, can be released in a reaction Exothermic – (Exo = out)(therm = heat) Reactions that release energy as light, heat or sound Endothermic – (Endo – enter) Reactions that absorb energy form the environment

Activation Energy • All reactions need some energy to begin
Catalysts • Catalysts are substances that increase the rate of a reaction, but are not used in the reaction • They lower the activation energy
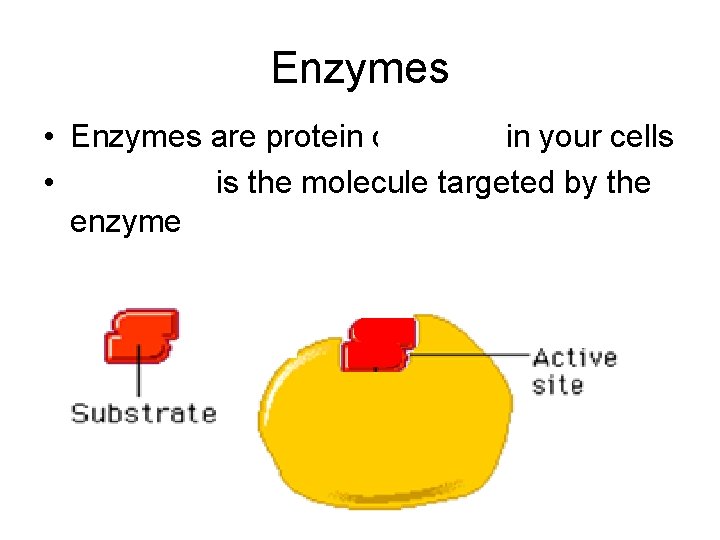
Enzymes • Enzymes are protein catalysts in your cells • Substrate is the molecule targeted by the enzyme
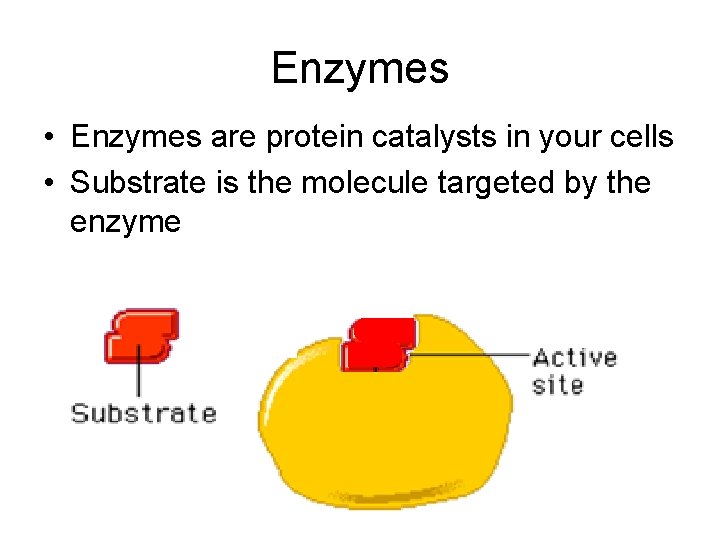
Enzymes • Enzymes are protein catalysts in your cells • Substrate is the molecule targeted by the enzyme
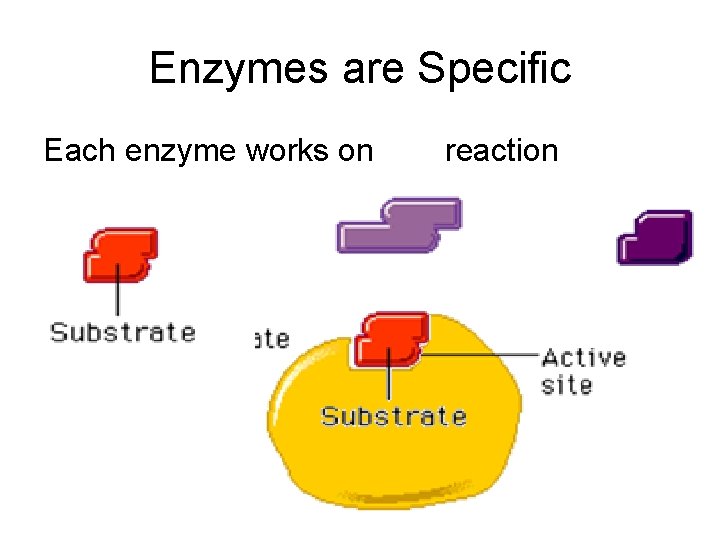
Enzymes are Specific Each enzyme works on one reaction
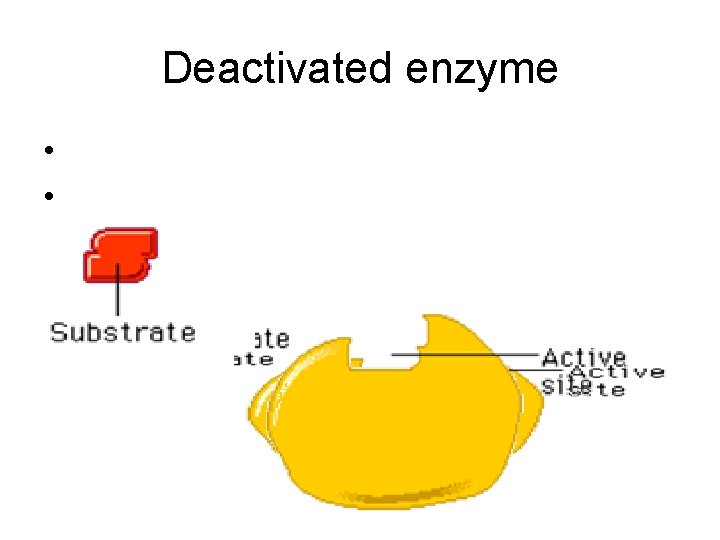
Deactivated enzyme • p. H • Temperature
- Slides: 78