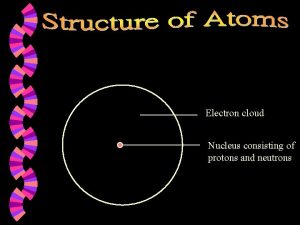
How to find the number of protons neutrons
How to find the number of protons, neutrons, & electrons for an element on the periodic table.
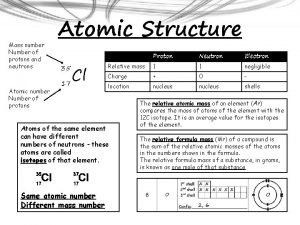
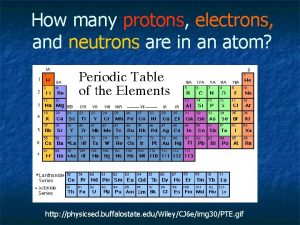
The periodic table is made up of elements. What is an element? An element is a substance that cannot be broken down into simpler substances by ordinary physical or chemical means. Elements are known as the building blocks of matter. It is made up of protons, neutrons, and electrons.

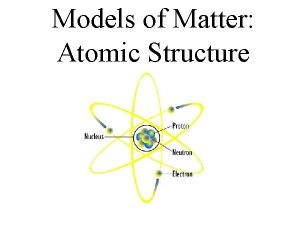
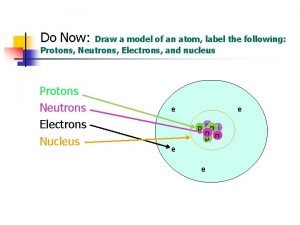
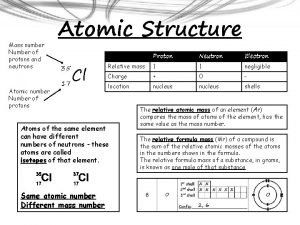
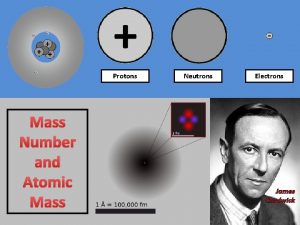
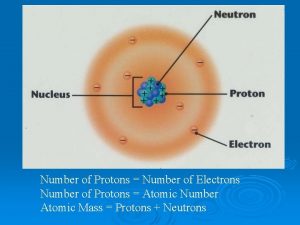
Protons have a positive charge, are located in the nucleus, and have a mass of 1 amu. Remember P+

Neutrons have a Neutral charge, are also located in the nucleus, and have a mass of 1 amu Remember N Isn’t this easy!
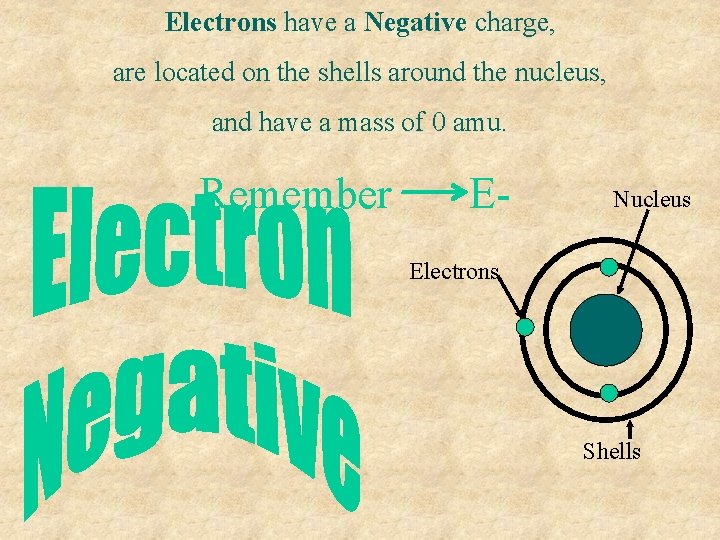
Electrons have a Negative charge, are located on the shells around the nucleus, and have a mass of 0 amu. Remember E- Nucleus Electrons Shells
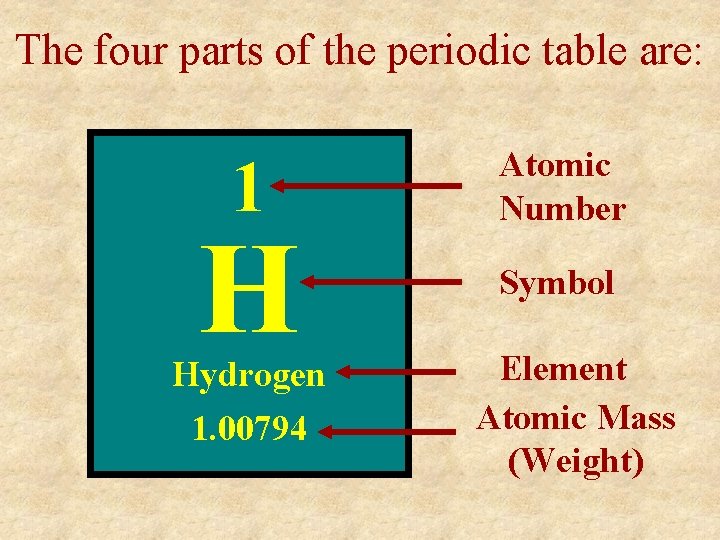
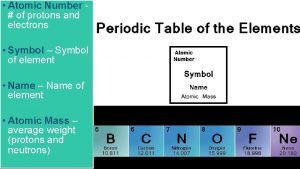
The four parts of the periodic table are: 1 H Hydrogen 1. 00794 Atomic Number Symbol Element Atomic Mass (Weight)

Quick Quiz What is the Atomic Number for Carbon (C)? What is the Atomic Number for Iron (Fe)? What is the Atomic Number for Potassium (K)? What is the Atomic Number for Nitrogen (N)? What is the Atomic Number for Gold (Au)?
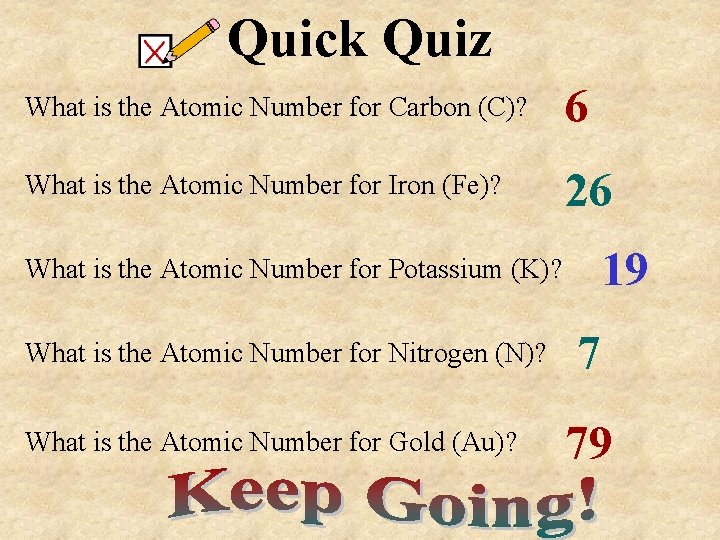
Quick Quiz What is the Atomic Number for Carbon (C)? 6 What is the Atomic Number for Iron (Fe)? 26 19 What is the Atomic Number for Potassium (K)? What is the Atomic Number for Nitrogen (N)? What is the Atomic Number for Gold (Au)? 7 79
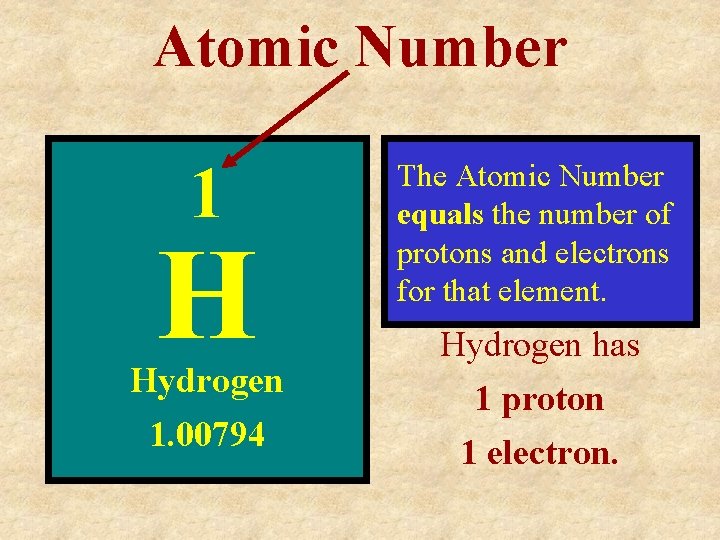
Atomic Number 1 H Hydrogen 1. 00794 The Atomic Number equals the number of protons and electrons for that element. Hydrogen has 1 proton 1 electron.
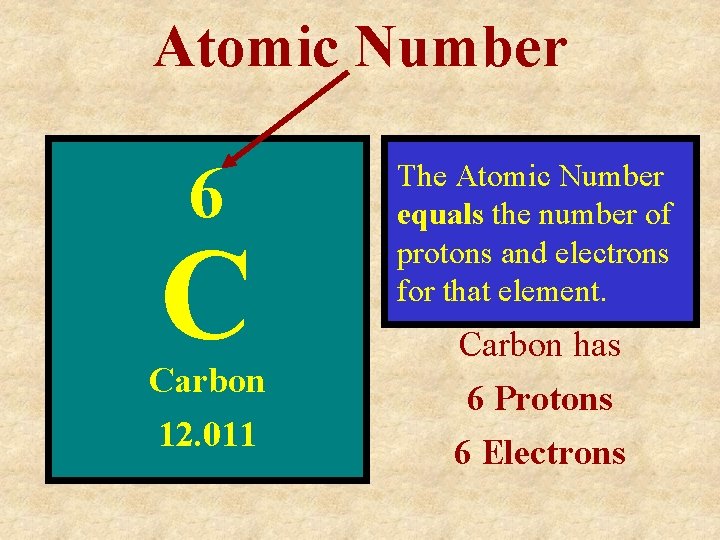
Atomic Number 6 C Carbon 12. 011 The Atomic Number equals the number of protons and electrons for that element. Carbon has 6 Protons 6 Electrons

Quick Quiz How many protons does Oxygen have? How many electrons does Oxygen have? How many protons does Calcium have? How many electrons does Calcium have? How many protons does Zinc have? How many electrons does Zinc have? How many protons does Tin (Sn) have? How many electrons does Tin (Sn) have? How many protons does Radon have? How many electrons does Radon have?

Quick Quiz How many protons does Oxygen have? How many electrons does Oxygen have? 8 How many protons does Calcium have? How many electrons does Calcium have? 20 How many protons does Zinc have? How many protons does Tin (Sn) have? How many protons does Radon have? 30 50 86

We’ve learned about protons and electrons. What are we missing?
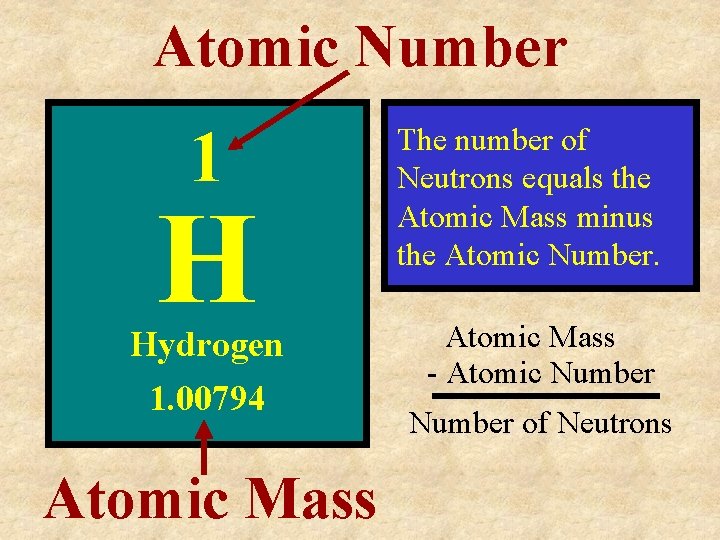
Atomic Number 1 H Hydrogen 1. 00794 Atomic Mass The number of Neutrons equals the Atomic Mass minus the Atomic Number. Atomic Mass - Atomic Number of Neutrons
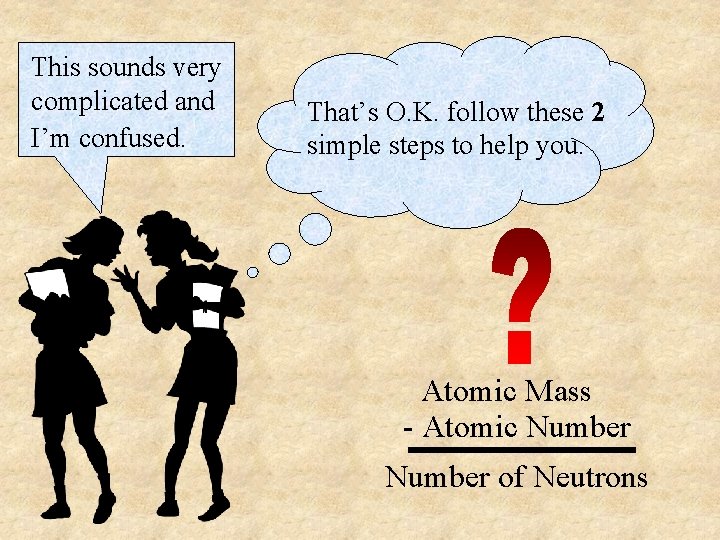
This sounds very complicated and I’m confused. That’s O. K. follow these 2 simple steps to help you. Atomic Mass - Atomic Number of Neutrons

Step 1 1 H Hydrogen 1. 00794 Atomic Mass Round the Atomic Mass to the nearest whole number. 1. 00794 Look at the tenths column. If the number in the tenths column is 5 or greater, then round the number up, if not leave unchanged. 1. 00794 rounded to the nearest whole number is?

Step 2 1 H Hydrogen 1. 00794 Find the Atomic Number: Take the rounded Atomic Mass Subtract the Atomic Number Equals the Number of Neutrons

Practice 7 N Nitrogen 14. 0067 Find the number of Neutrons in Nitrogen: Take the rounded Atomic Mass Subtract the Atomic Number Equals the Number of Neutrons

Practice 2 17 Cl Chlorine 35. 453 Find the number of Neutrons in Chlorine: Take the rounded Atomic Mass Subtract the Atomic Number Equals the Number of Neutrons

Practice 2 17 Cl Chlorine 35. 453 Find the number of Neutrons in Chlorine: Take the rounded Atomic Mass Subtract the Atomic Number Equals the Number of Neutrons

Practice 3 79 Au Gold 196. 9665 Find the number of Neutrons in Gold: Take the rounded Atomic Mass Subtract the Atomic Number Equals the Number of Neutrons

Practice 3 79 Au Gold 196. 9665 Find the number of Neutrons in Gold: Take the rounded Atomic Mass Subtract the Atomic Number Equals the Number of Neutrons
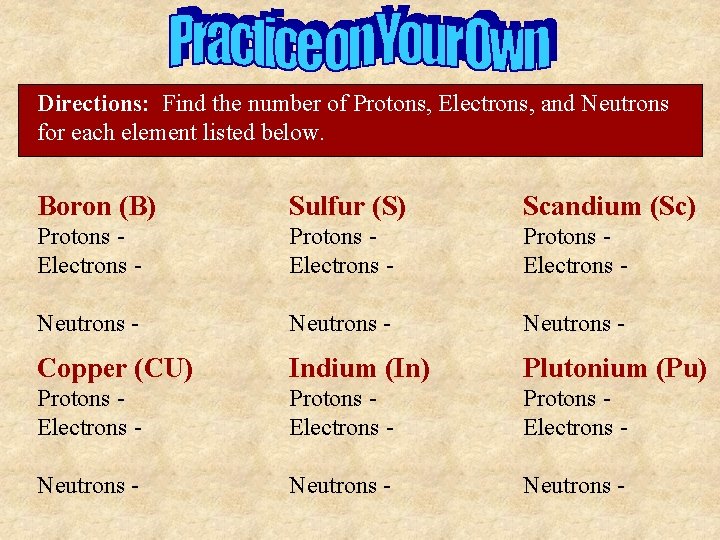
Directions: Find the number of Protons, Electrons, and Neutrons for each element listed below. Boron (B) Sulfur (S) Scandium (Sc) Protons Electrons - Neutrons - Copper (CU) Indium (In) Plutonium (Pu) Protons Electrons - Neutrons -
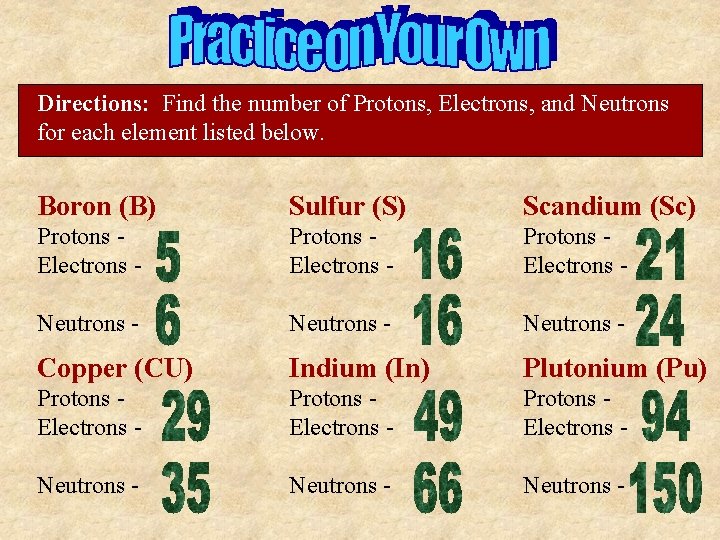
Directions: Find the number of Protons, Electrons, and Neutrons for each element listed below. Boron (B) Sulfur (S) Scandium (Sc) Protons Electrons - Neutrons - Copper (CU) Indium (In) Plutonium (Pu) Protons Electrons - Neutrons -

Now you know a little about the periodic table and how to find the number of protons, electrons, and neutrons.
- Slides: 25