Neutrons 101 Properties of Neutrons What is a






![X-rays and Neutrons - Difference 2 • l~10 -10 m [Å] (for both neutrons X-rays and Neutrons - Difference 2 • l~10 -10 m [Å] (for both neutrons](https://slidetodoc.com/presentation_image_h/8fe23a9d3c735e59befd86f2ab7f084d/image-33.jpg)

- Slides: 36

Neutrons 101 Properties of Neutrons

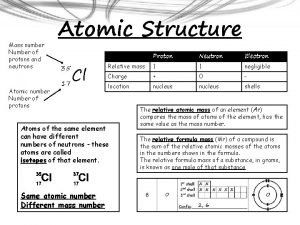
What is a neutron? • The neutron is a subatomic particle with no net electric charge. Nucleus • Neutrons are usually bound (via strong nuclear force) in atomic nuclei. Nuclei consist of protons and neutrons—both known as nucleons. • The number of protons determines the element & the number of neutrons determines the isotope, e. g. 15 N and 14 N have 7 p and 8 n and 7 n respectively.

Instability of free neutron and mass • Free neutrons are unstable; they undergo b-decay, lifetime ~ 885. 7 ± 0. 8 s. • They cannot be stored for long free! • n 0 → p+ + e− + νe • Mass is slightly larger than that of a proton

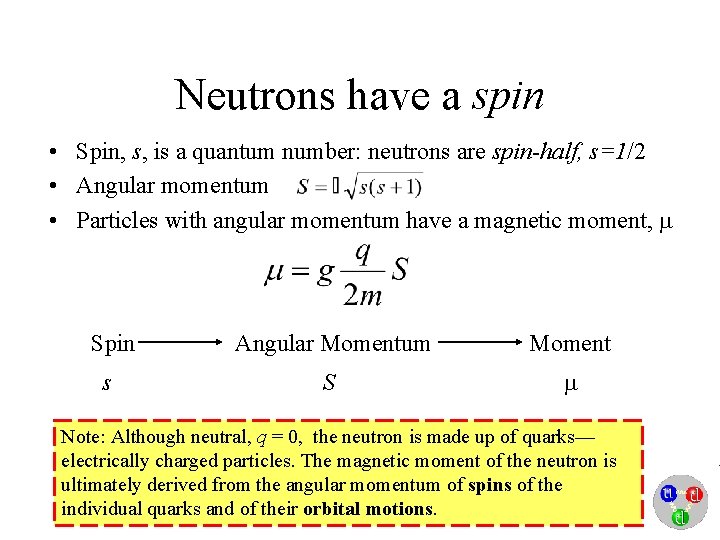
Neutrons have a spin • Spin, s, is a quantum number: neutrons are spin-half, s=1/2 • Angular momentum • Particles with angular momentum have a magnetic moment, Spin Angular Momentum Moment s S Note: Although neutral, q = 0, the neutron is made up of quarks— electrically charged particles. The magnetic moment of the neutron is ultimately derived from the angular momentum of spins of the individual quarks and of their orbital motions.

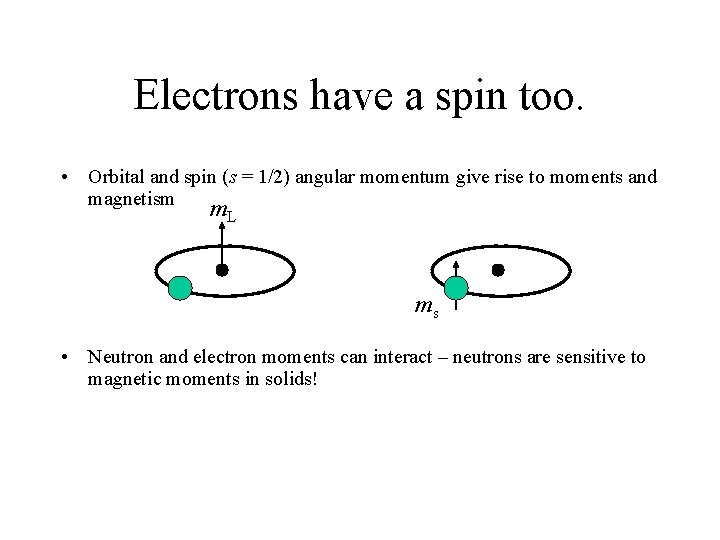
Electrons have a spin too. • Orbital and spin (s = 1/2) angular momentum give rise to moments and magnetism m. L ms • Neutron and electron moments can interact – neutrons are sensitive to magnetic moments in solids!

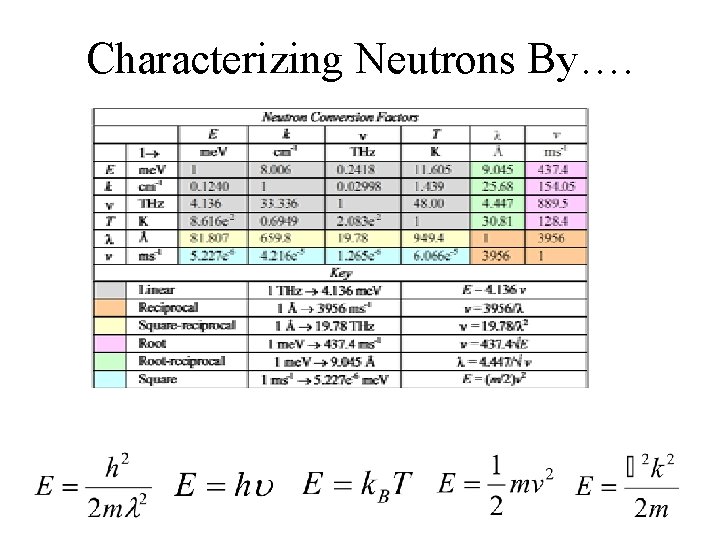
Characterizing Neutrons By….

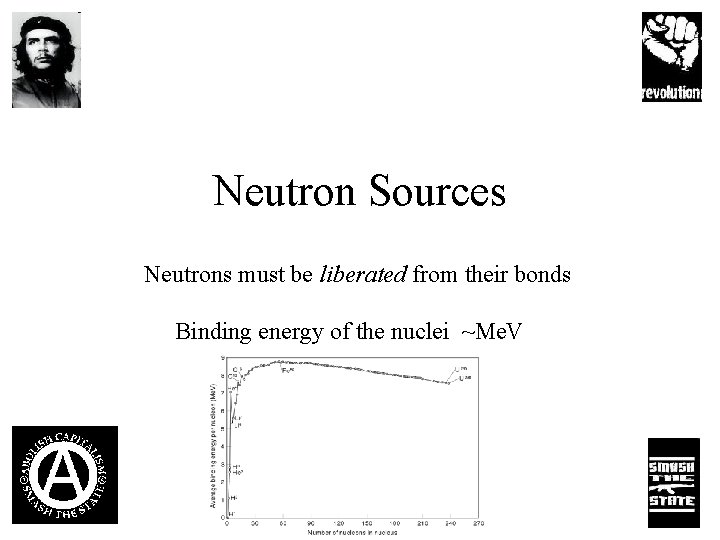
Neutron Sources Neutrons must be liberated from their bonds Binding energy of the nuclei ~Me. V

a-particles with light elements Discovery of the Neutron • Neutrons are produced when a-particles hit 1930 Waltherlow-Z Bothe and H. Beckerincluding found that a-particles several isotopes those of emitted from Po fell on certain light elements a highly Be, C, O. As an example, a representative a penetrating radiation was produced: (a, n). -Be neutron source produces ~30 neutrons 1932 Irène Joliot-Curie anda-particles. Frédéric Joliot showed that if for every million this unknown radiation fell on hydrogenous compounds it • e. g. , very Pu. Be. ejected high-energy protons (n, p). 1932 James Chadwick showed that the g-ray hypothesis was untenable and that the new radiation was uncharged particles of approximately the mass of the proton.

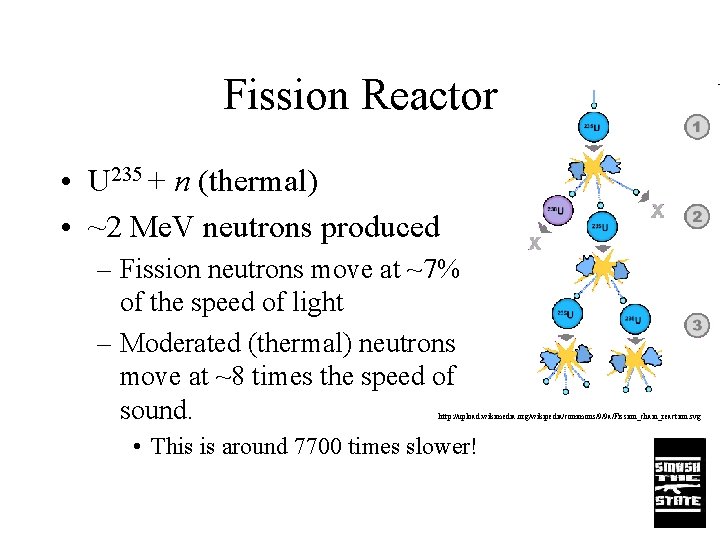
Fission Reactor • U 235 + n (thermal) • ~2 Me. V neutrons produced – Fission neutrons move at ~7% of the speed of light – Moderated (thermal) neutrons move at ~8 times the speed of sound. http: //upload. wikimedia. org/wikipedia/commons/9/9 a/Fission_chain_reaction. svg • This is around 7700 times slower!

Spallation Source • Spallation=“blowing chunks” (p, n) • hydride ion (H-) source proton accelerators targets moderators instruments http: //www. isis. rl. ac. uk/

Moderation/Slowing-down -neutrons as particles (“gas”)

Maxwellian • Distribution of velocities of particles as f(T) – neutrons behave like a gas. • Maxwell-Boltzmann distribution-the most probable speed distribution in a collisionallydominated system consisting of a large number of non-interacting particles. – describes the neutron spectrum to a good approximation (ignoring l-dependent absorption).

Moderators • Light nuclei + low absorption. An collision is a collision in whichthe nucleus total kineticand • elastic Elastic* collisions between energy of the colliding bodies after collision is equal to their neutron transfer energy. totalthe kinetic energy before collision. * • Moderated neutrons take on the average kinetic energy of the moderator, set by its T. How many collisions are necessary to moderate a 2 Me. V fission neutron to a 1 e. V neutron? ~16 for light water, which take place in about 30 cm of travel. Simon Steinmann, Raul Roque: Creative Commons Attribution Share. Alike 2. 5

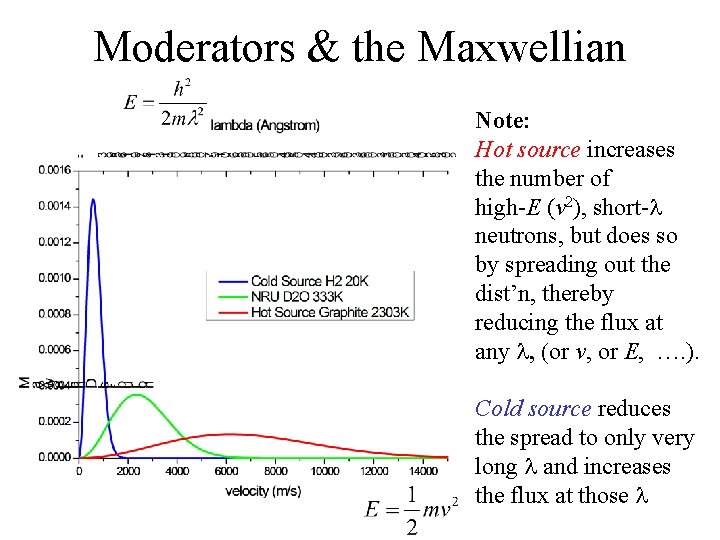
Moderators & the Maxwellian Note: Hot source increases the number of high-E (v 2), short-l neutrons, but does so by spreading out the dist’n, thereby reducing the flux at any l, (or v, or E, …. ). Cold source reduces the spread to only very long l and increases the flux at those l

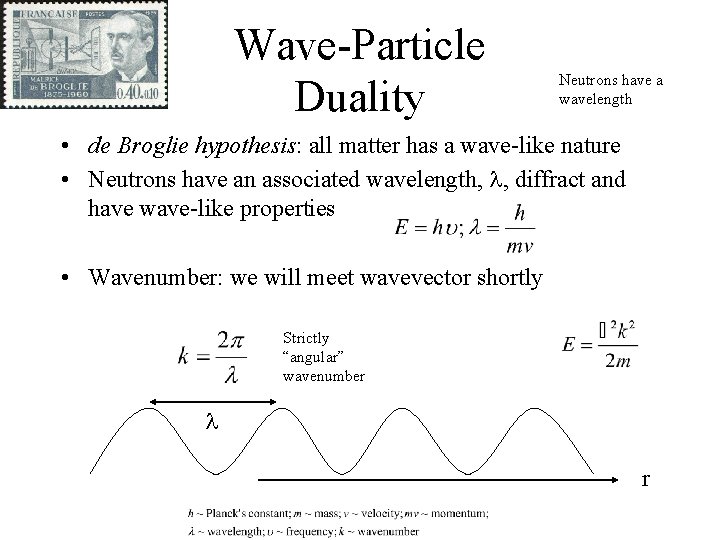
Wave-Particle Duality Neutrons have a wavelength • de Broglie hypothesis: all matter has a wave-like nature • Neutrons have an associated wavelength, l, diffract and have wave-like properties • Wavenumber: we will meet wavevector shortly Strictly “angular” wavenumber l r

Waves http: //upload. wikimedia. org/wikipedia/commons/5/5 c/Plane_wave. gif http: //upload. wikimedia. org/wikipedia/commons/1/12/Spherical_wave 2. gif

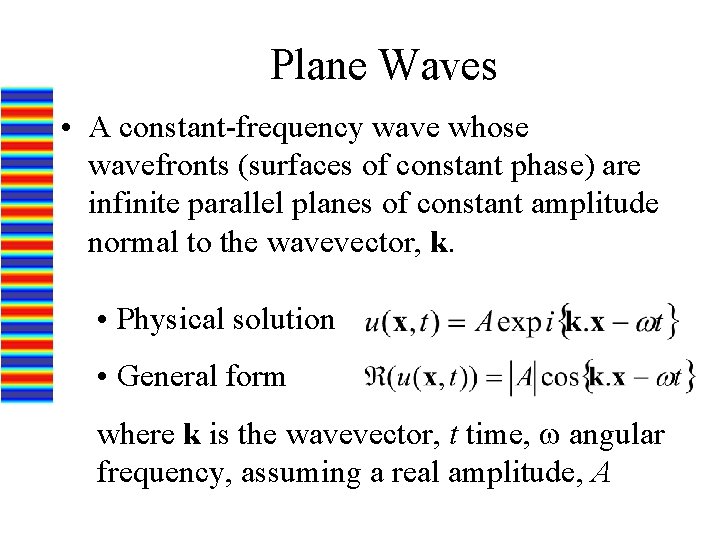
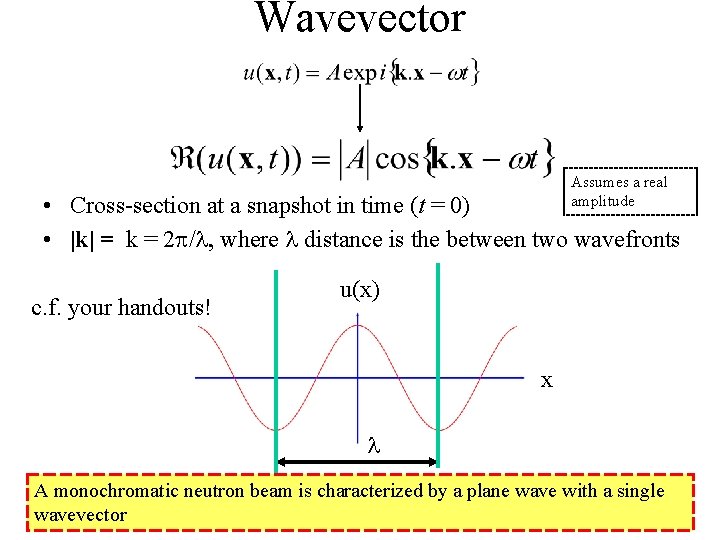
Plane Waves • A constant-frequency wave whose wavefronts (surfaces of constant phase) are infinite parallel planes of constant amplitude normal to the wavevector, k. • Physical solution • General form where k is the wavevector, t time, w angular frequency, assuming a real amplitude, A

Wavevector Assumes a real amplitude • Cross-section at a snapshot in time (t = 0) • |k| = k = 2 p/l, where l distance is the between two wavefronts c. f. your handouts! u(x) x l A monochromatic neutron beam is characterized by a plane wave with a single wavevector

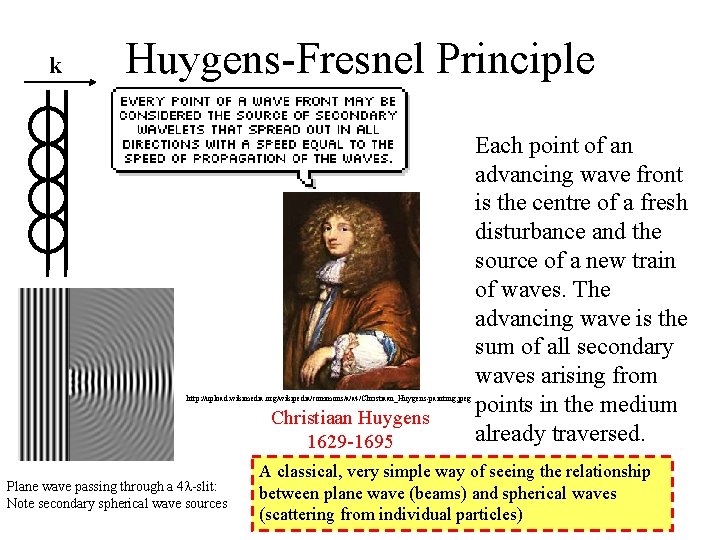
k Huygens-Fresnel Principle http: //upload. wikimedia. org/wikipedia/commons/a/a 4/Christiaan_Huygens-painting. jpeg Christiaan Huygens 1629 -1695 Plane wave passing through a 4 l-slit: Note secondary spherical wave sources Each point of an advancing wave front is the centre of a fresh disturbance and the source of a new train of waves. The advancing wave is the sum of all secondary waves arising from points in the medium already traversed. A classical, very simple way of seeing the relationship between plane wave (beams) and spherical waves (scattering from individual particles)

Ocean plane waves passing through slits http: //www. physics. gatech. edu/gcuo/Ultrafast. Optics/Optics. I/lectures/Optics. I-20 -Diffraction-I. ppt

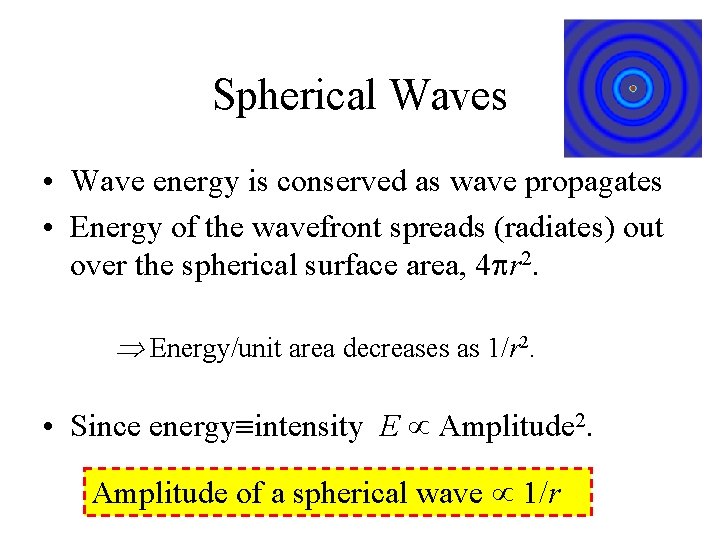
Spherical Waves • Wave energy is conserved as wave propagates • Energy of the wavefront spreads (radiates) out over the spherical surface area, 4 pr 2. Energy/unit area decreases as 1/r 2. • Since energy intensity E Amplitude 2. Amplitude of a spherical wave 1/r

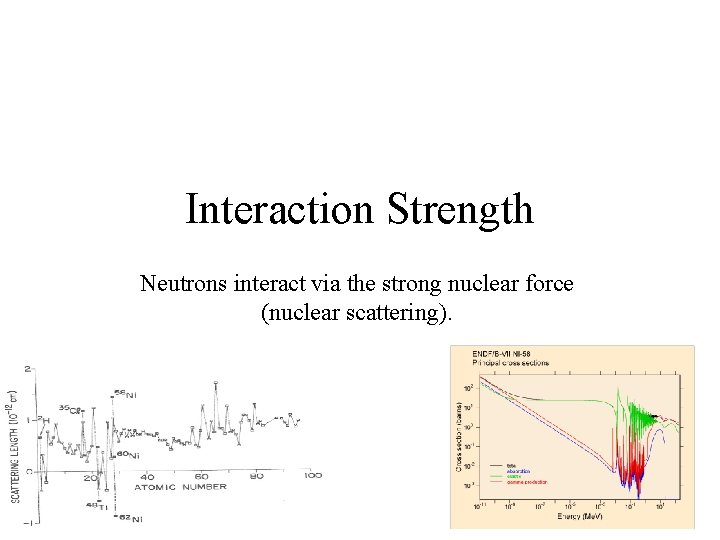
Interaction Strength Neutrons interact via the strong nuclear force (nuclear scattering).

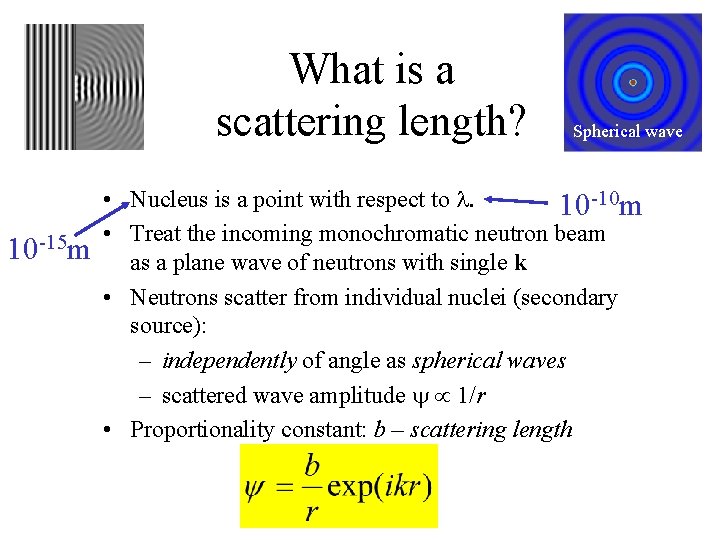
What is a scattering length? 10 -15 m Spherical wave • Nucleus is a point with respect to l. 10 -10 m • Treat the incoming monochromatic neutron beam as a plane wave of neutrons with single k • Neutrons scatter from individual nuclei (secondary source): – independently of angle as spherical waves – scattered wave amplitude 1/r • Proportionality constant: b – scattering length

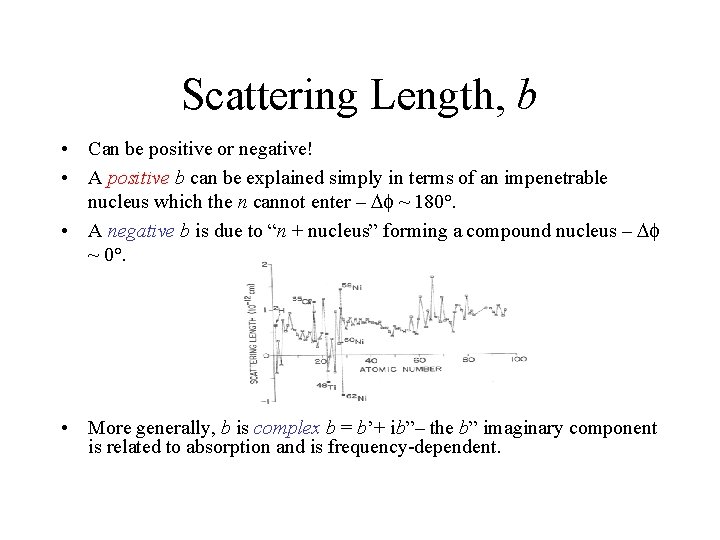
Scattering Length, b • Can be positive or negative! • A positive b can be explained simply in terms of an impenetrable nucleus which the n cannot enter – D ~ 180°. • A negative b is due to “n + nucleus” forming a compound nucleus – D ~ 0°. • More generally, b is complex b = b’+ ib”– the b” imaginary component is related to absorption and is frequency-dependent.

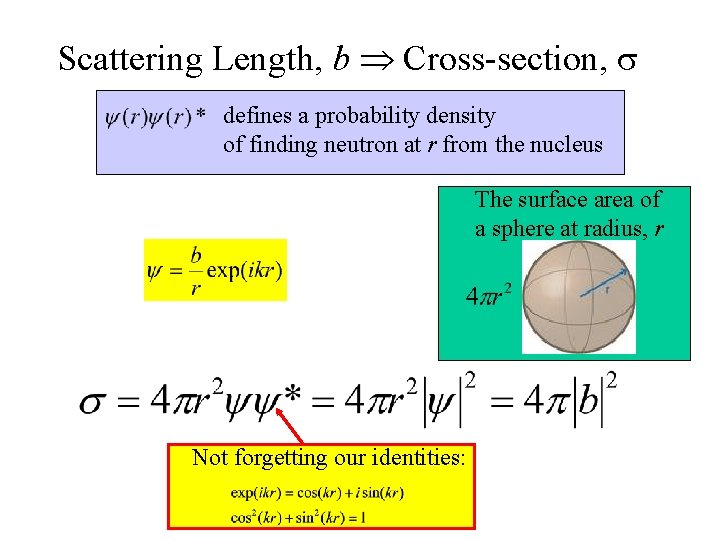
Scattering Length, b Cross-section, s defines a probability density of finding neutron at r from the nucleus The surface area of a sphere at radius, r Not forgetting our identities:

Cross-section U is “as big as a barn. ” • The interaction probability is the likelihood of a point-projectile hitting the target area (the cross section, σ). • Each nucleus thought of as being surrounded by a a characteristic area. • Barn = 10− 28 m 2, ~ the cross sectional area of U. • Cross-sections for different processes: scattering, absorption, fission… • They are not constant, but energy-dependent There also units of sheds, and outhouses…but not used for neutrons….

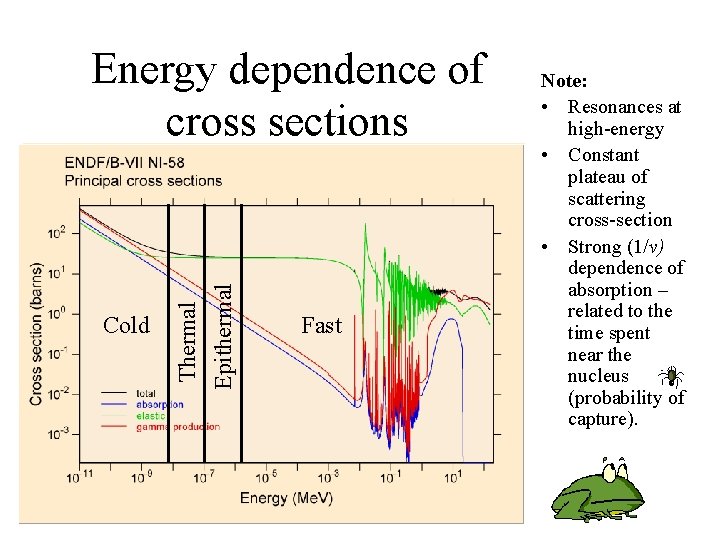
Cold Thermal Epithermal Energy dependence of cross sections Fast Note: • Resonances at high-energy • Constant plateau of scattering cross-section • Strong (1/v) dependence of absorption – related to the time spent near the nucleus (probability of capture).

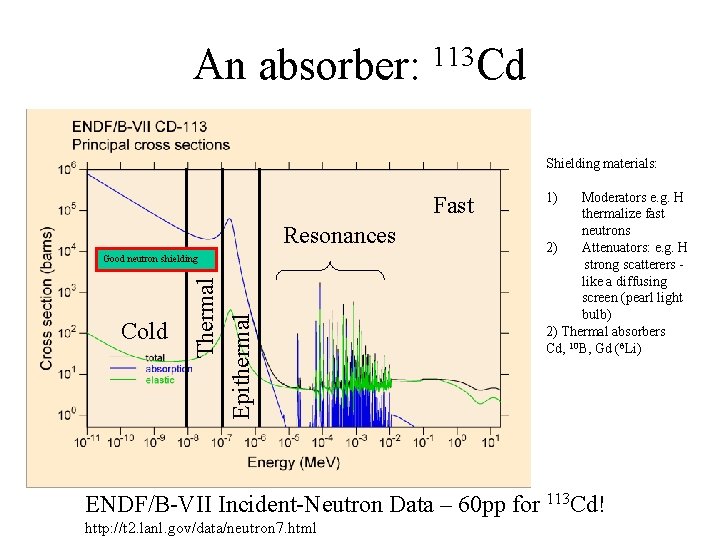
An absorber: 113 Cd Shielding materials: Fast Resonances Cold Thermal Epithermal Good neutron shielding 1) Moderators e. g. H thermalize fast neutrons 2) Attenuators: e. g. H strong scatterers like a diffusing screen (pearl light bulb) 2) Thermal absorbers Cd, 10 B, Gd (6 Li) ENDF/B-VII Incident-Neutron Data – 60 pp for 113 Cd! http: //t 2. lanl. gov/data/neutron 7. html

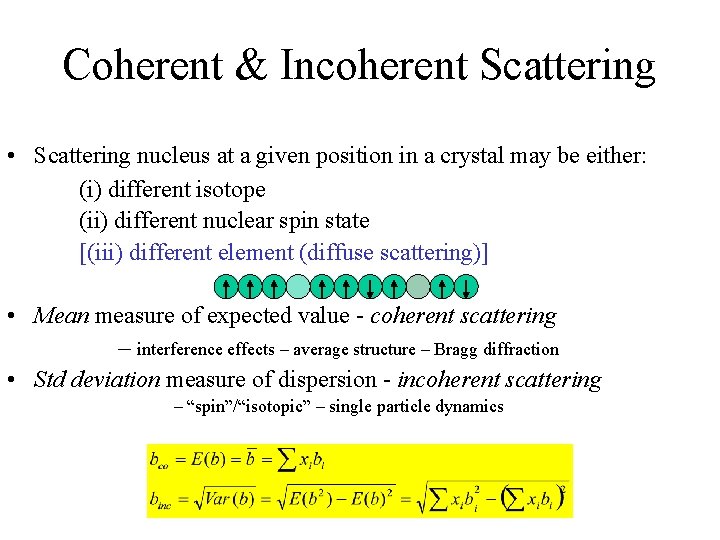
Coherent & Incoherent Scattering • Scattering nucleus at a given position in a crystal may be either: (i) different isotope (ii) different nuclear spin state [(iii) different element (diffuse scattering)] • Mean measure of expected value - coherent scattering – interference effects – average structure – Bragg diffraction • Std deviation measure of dispersion - incoherent scattering – “spin”/“isotopic” – single particle dynamics

. . which leads to comparison to Xray scattering

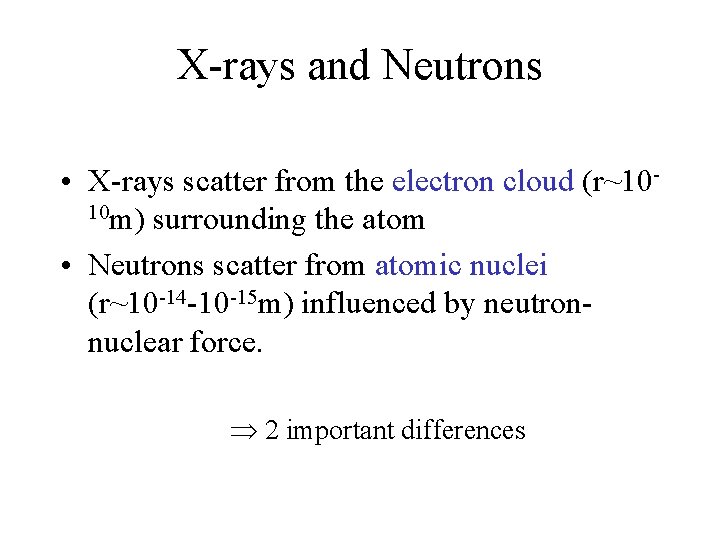
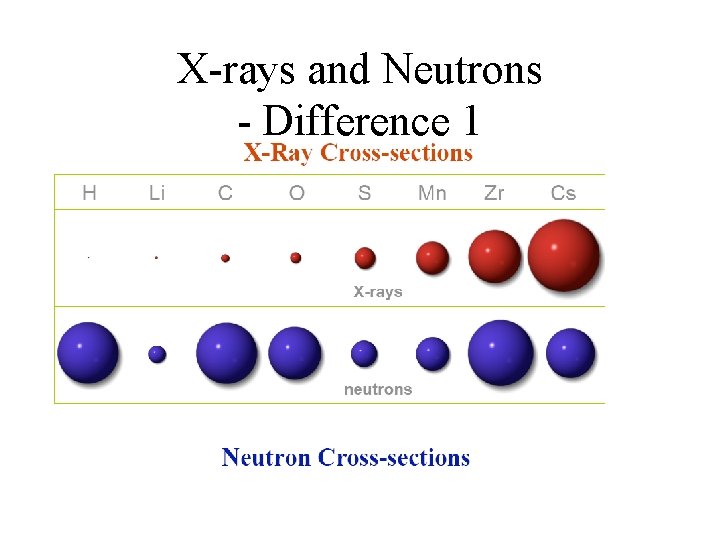
X-rays and Neutrons • X-rays scatter from the electron cloud (r~1010 m) surrounding the atom • Neutrons scatter from atomic nuclei (r~10 -14 -10 -15 m) influenced by neutronnuclear force. 2 important differences

X-rays and Neutrons - Difference 1 • X-rays scatter from the electron cloud: ss Z 2. • Neutrons scatter from atomic nuclei: ss ~ isotope-dependent
![Xrays and Neutrons Difference 2 l10 10 m Å for both neutrons X-rays and Neutrons - Difference 2 • l~10 -10 m [Å] (for both neutrons](https://slidetodoc.com/presentation_image_h/8fe23a9d3c735e59befd86f2ab7f084d/image-33.jpg)
X-rays and Neutrons - Difference 2 • l~10 -10 m [Å] (for both neutrons and X-rays) • X-rays scatter from the electron cloud (r~10 -10 m) [Å] • Neutrons scatter from atomic nuclei (r~10 -14 -10 -15 m) [fm] Four orders of magnitude: Nucleus: l is as Deep-River—Pembroke: Earth—Moon Nuclei are point scatterers wrt l.

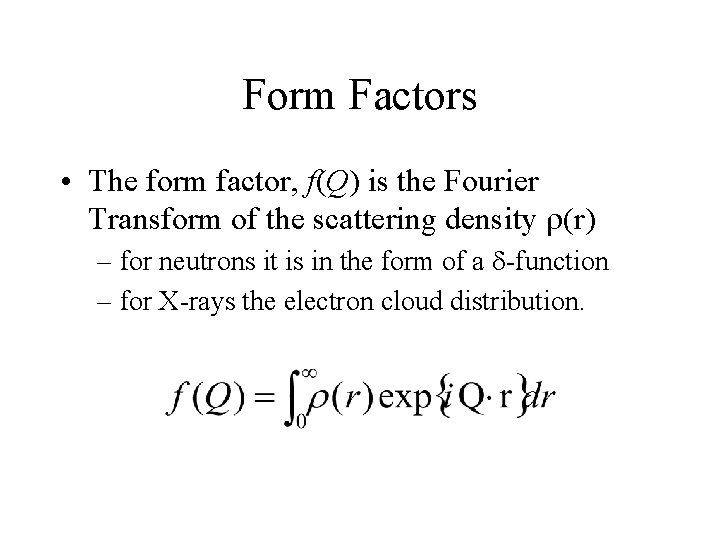
Form Factors • The form factor, f(Q) is the Fourier Transform of the scattering density r(r) – for neutrons it is in the form of a d-function – for X-rays the electron cloud distribution.

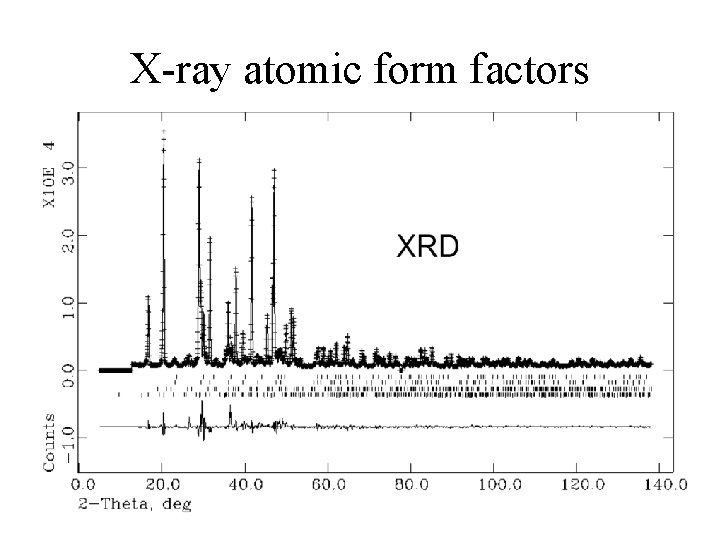
X-ray atomic form factors Low angles, little path difference 10 -12 cm 5 4 3 2 1 High angles, greater path difference X-ray Neutron 1 (Sin q)/l 108 cm-1 K-atom X-ray: Destructive interference is possible at high angles due to finite size of electron cloud form factor Neutron: Nucleus is orders of magnitude smaller than neutron wavelength no form factor

Summary • • • Spin, charge etc Energy, velocity, wavelength Moderation Cross section, scattering length X-rays vs. neutrons
 Intensive property and extensive properties
Intensive property and extensive properties Chemical properties of citric acid
Chemical properties of citric acid 39k+ protons neutrons electrons
39k+ protons neutrons electrons Can an atom have more neutrons than protons
Can an atom have more neutrons than protons Number of neutrons helium
Number of neutrons helium Sonda de neutrons
Sonda de neutrons Period 5 transition metal 51 neutrons
Period 5 transition metal 51 neutrons Can an atom have more neutrons than protons
Can an atom have more neutrons than protons Lithium protons neutrons electrons
Lithium protons neutrons electrons Entdeckung des neutrons
Entdeckung des neutrons U 238 protons neutrons electrons
U 238 protons neutrons electrons I am the first element in the fourth period
I am the first element in the fourth period Atomic structure jeopardy
Atomic structure jeopardy How to find electrons on periodic table
How to find electrons on periodic table Neutron star
Neutron star Colbot-60 has how many neutrons?
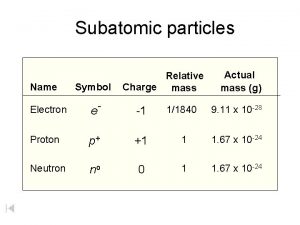
Colbot-60 has how many neutrons? Subatomic particles chart
Subatomic particles chart Lithium number of protons and neutrons
Lithium number of protons and neutrons Neutrons
Neutrons Bc atomic mass
Bc atomic mass Symbol for subatomic particles
Symbol for subatomic particles How many neutrons does silicon 29 have
How many neutrons does silicon 29 have Neutrons in hydrogen
Neutrons in hydrogen Calcium metal or nonmetal
Calcium metal or nonmetal Find the number of protons c
Find the number of protons c 24mg12 2+
24mg12 2+ Which equation represents sublimation?
Which equation represents sublimation? Lithium mass number
Lithium mass number Istopos
Istopos 39k+ protons neutrons electrons
39k+ protons neutrons electrons Mass of protons neutrons electrons
Mass of protons neutrons electrons Whats the atomic mass for boron
Whats the atomic mass for boron Chromium 58 protons, neutrons electrons
Chromium 58 protons, neutrons electrons Element number 70
Element number 70 Xenon neutrons
Xenon neutrons How many neutrons does the isotope n-14 have
How many neutrons does the isotope n-14 have Protons and neutrons size
Protons and neutrons size