What has a charge of 1 Which scientist
















- Slides: 21


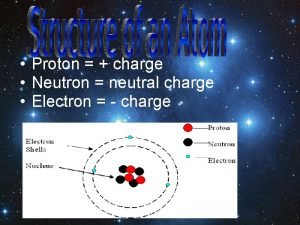
What has a charge of +1?

Which scientist developed the plum pudding model of the atom?

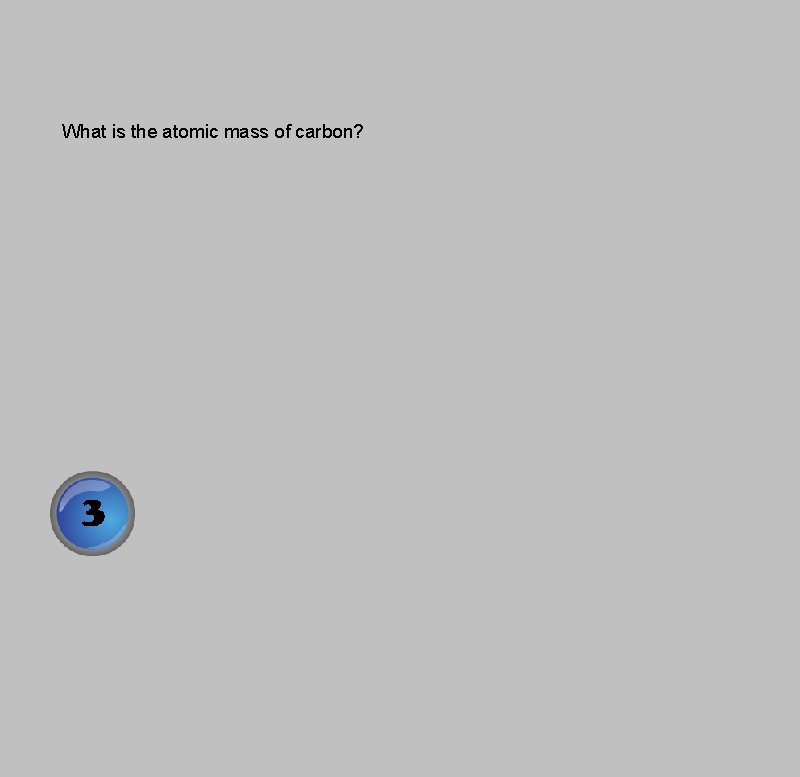
What is the atomic mass of carbon?

What is the number of neutrons in Bromine-80?

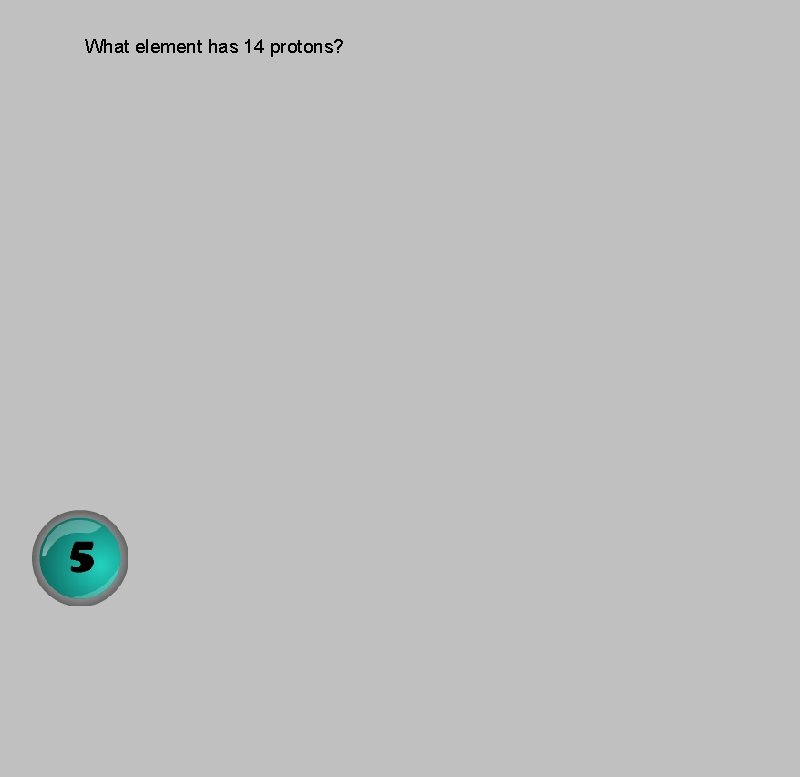
What element has 14 protons?

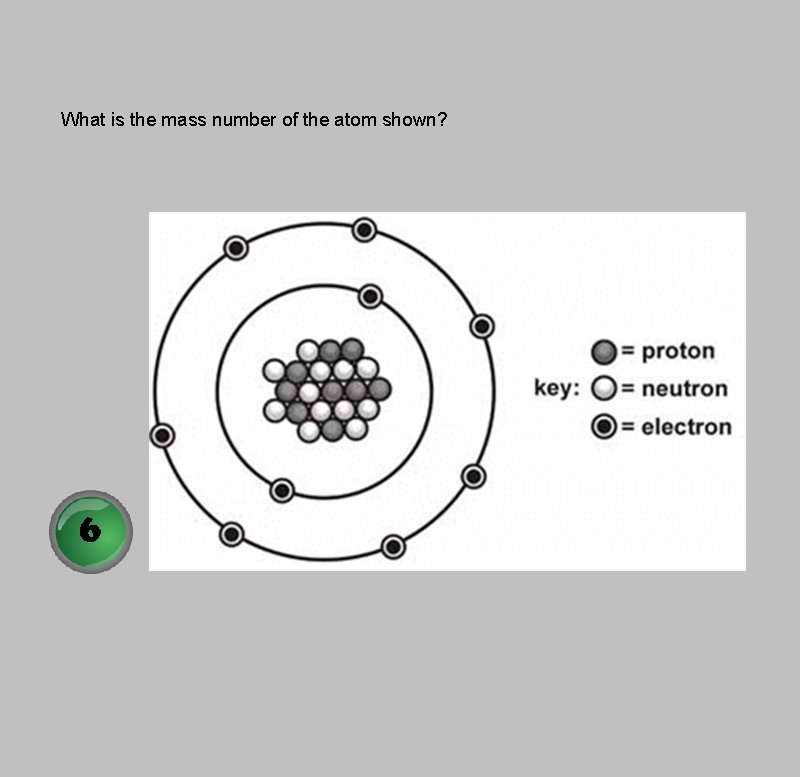
What is the mass number of the atom shown?

How many neutrons are in an atom of chlorine?

Which one is comprised of the other three? a. proton b. atom c. electron d. neutron

Rutherford expected alpha particle to travel almost straight through a target of gold foil. The results of his gold foil experiment did not support a. Millikan’s oil drop experiment b. Thomson’s plum pudding theory c. the cathode ray phenomenon d. Bohr’s atomic model

What does it mean when two atoms are isotopes of one another?

An atom has 23 protons and 29 neutrons. What is the correct chemical symbol for this atom?

An industrially important element contains 26 electrons and rusts in the presence of air and moisture. Identify the element.

What element has 10 protons?

Magnesium has three isotopes. Magnesium-24 has a percent abundance of 78. 99%. Magnesium-26 has a percent abundance of 11. 01%. What is the percent abundance of magnesium-25?

Calculate the atomic mass of iridium. Iridium has two isotopes. Iridium-191 has a mass of 191. 0 amu and a percent abundance of 37. 58%. Iridium-193 has a mass of 193. 0 amu and a percent abundance of 62. 42%.

How many more neutrons does thorium-230 have than protons? How many electrons does thorium-230 have?

How many protons, electrons, and neutrons are in U-235?

How many protons, electrons, and neutrons are in H-3?

How many protons, electrons, and neutrons are in silicon-29?

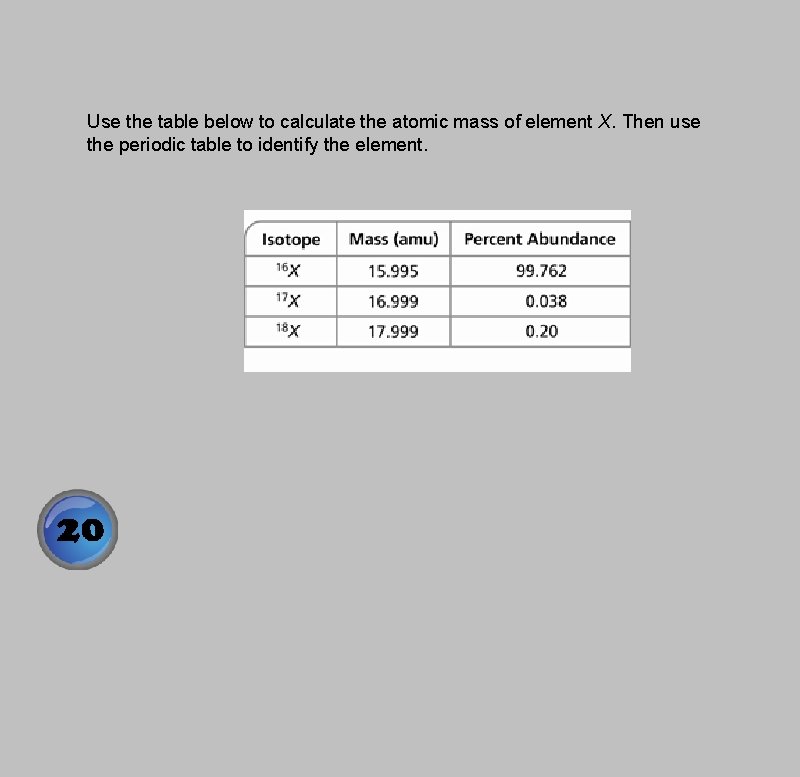
Use the table below to calculate the atomic mass of element X. Then use the periodic table to identify the element.
 Gamma decay equation
Gamma decay equation Which scientist developed the quantum mechanical
Which scientist developed the quantum mechanical Which scientist
Which scientist Which scientist
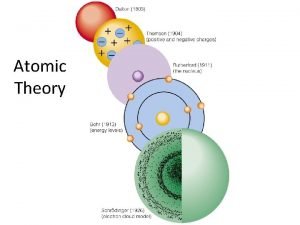
Which scientist Name of atom
Name of atom An industrially important element contains 26
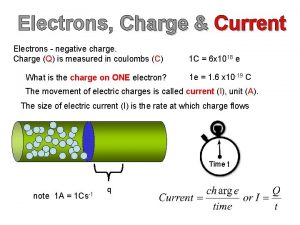
An industrially important element contains 26 Difference between charge and electric charge
Difference between charge and electric charge Difference between static and current electricity
Difference between static and current electricity A charm quark has a charge of approximately
A charm quark has a charge of approximately A rod of length l has a uniform positive charge
A rod of length l has a uniform positive charge Food scientists measure food energy in
Food scientists measure food energy in Data scientist ausbildung wien
Data scientist ausbildung wien Things a computer scientist rarely talks about
Things a computer scientist rarely talks about Scientists hypothesize that cabbage broccoli
Scientists hypothesize that cabbage broccoli Making of a scientist mind map
Making of a scientist mind map Far side rocket scientist
Far side rocket scientist Staff scientist nih
Staff scientist nih Si derived unit of pressure scientist
Si derived unit of pressure scientist Mad scientist club book
Mad scientist club book Penjual sebagai artis dan scientist
Penjual sebagai artis dan scientist Forensic is derived from the latin word
Forensic is derived from the latin word What makes a scientist
What makes a scientist