Which scientist developed the quantum mechanical model of








![ANSWERS A) B) C) D) Nb (41) At (85) Ag (47) Cm (96) [Kr] ANSWERS A) B) C) D) Nb (41) At (85) Ag (47) Cm (96) [Kr]](https://slidetodoc.com/presentation_image_h/8858d7b59e81f4306f908d439e9bbb88/image-21.jpg)






- Slides: 31

Which scientist developed the quantum mechanical model of the atom? A. ) Bohr B. ) Rutherford C. ) Schrodinger D. ) Heisenberg

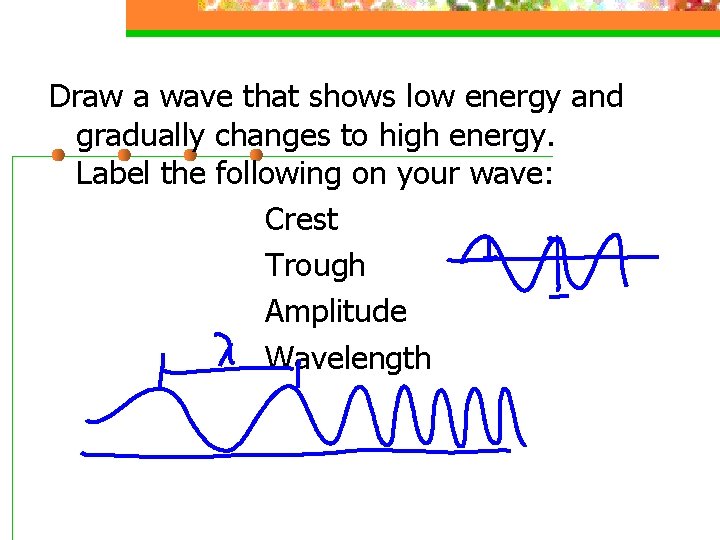
Draw a wave that shows low energy and gradually changes to high energy. Label the following on your wave: Crest Trough Amplitude Wavelength

Which of the following orbitals has the lowest energy? A) B) C) D) 4 p 4 s 3 d 4 f

The formula 2 n 2 represents _____. A) B) C) D) The number of sublevels in any energy level The maximum number of electrons that can occupy an energy level The number of orbitals None of the above

What type of atomic orbitals are in the third energy level? A) B) C) D) p and d only s, p, d, and f s and p only s, p, and d only

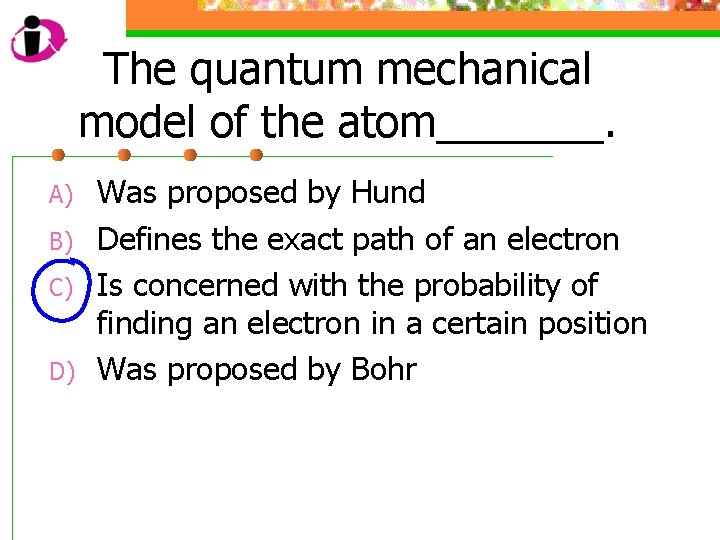
The quantum mechanical model of the atom_______. A) B) C) D) Was proposed by Hund Defines the exact path of an electron Is concerned with the probability of finding an electron in a certain position Was proposed by Bohr

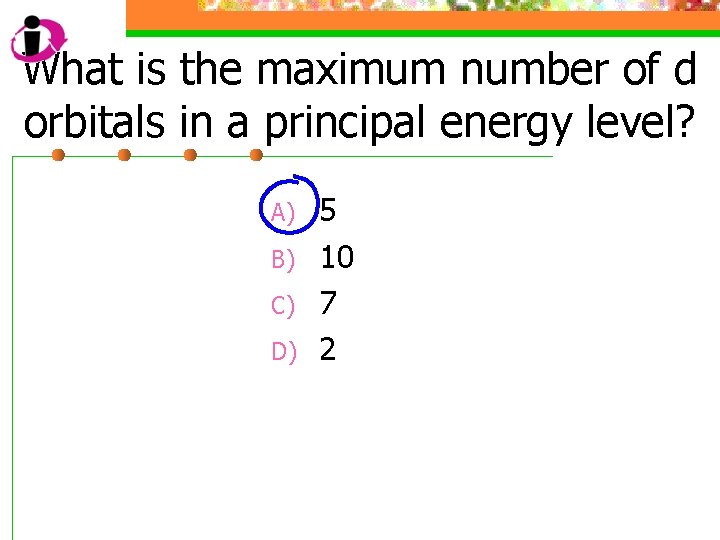
What is the maximum number of d orbitals in a principal energy level? A) B) C) D) 5 10 7 2

Write the complete electron configuration for titanium.

The principal quantum number indicates what property of an electron? A) B) C) D) Speed Orbital shape Spin Energy level

Which one of the following electron configurations is most stable? A) B) C) D) 5 s 1 5 s 3 5 s 2 5 s 4 4 d 5 4 d 3 4 d 4 4 d 2

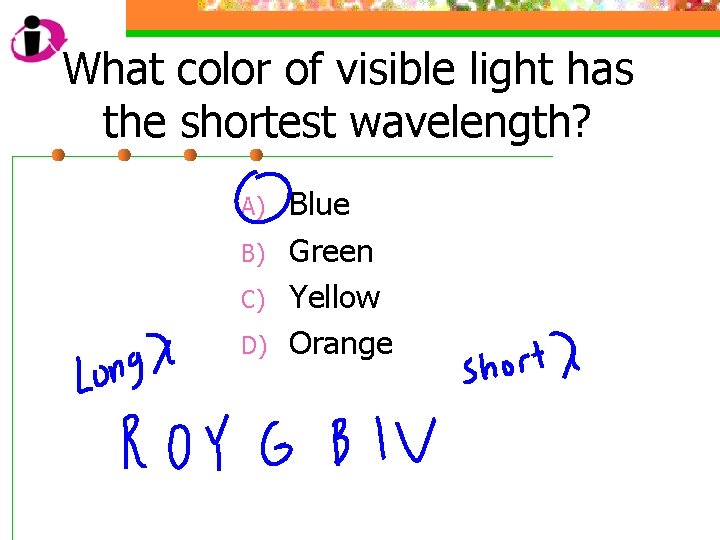
What color of visible light has the shortest wavelength? A) B) C) D) Blue Green Yellow Orange

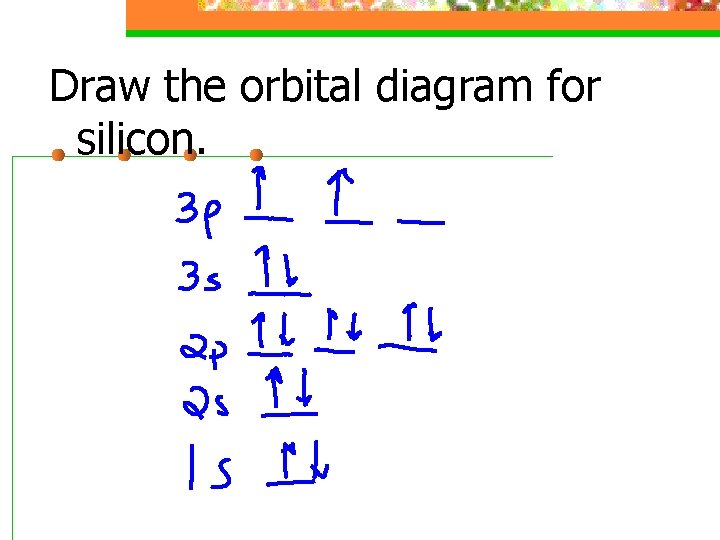
Draw the orbital diagram for silicon.

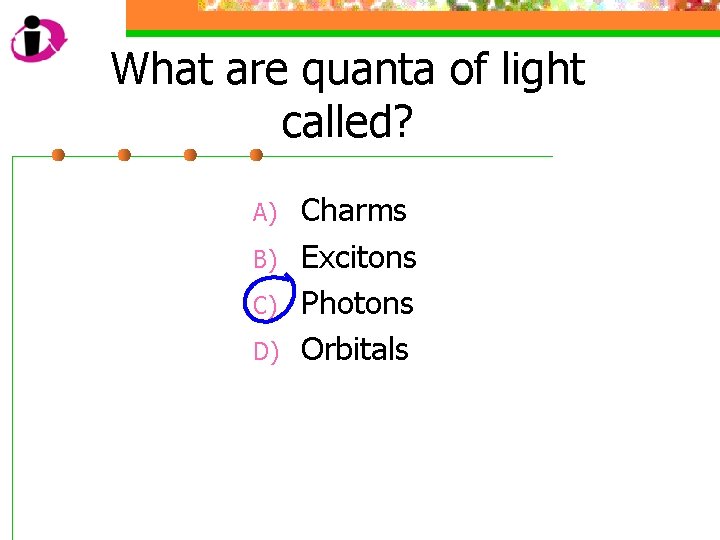
What are quanta of light called? A) B) C) D) Charms Excitons Photons Orbitals

The atomic emission spectra of a sodium atom on earth and of a sodium atom in the sun would be _______. A) B) C) D) The same as each other only in UV range The same as those of several other elements Different from each other

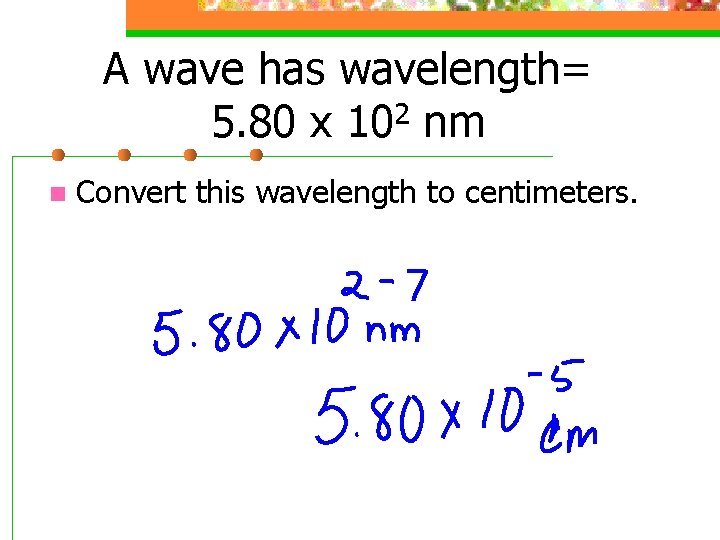
A wave has wavelength= 2 5. 80 x 10 nm n Convert this wavelength to centimeters.

The lowest energy state of an atom is called the ______. A) B) C) D) Excited state Dependent state Independent state Ground state

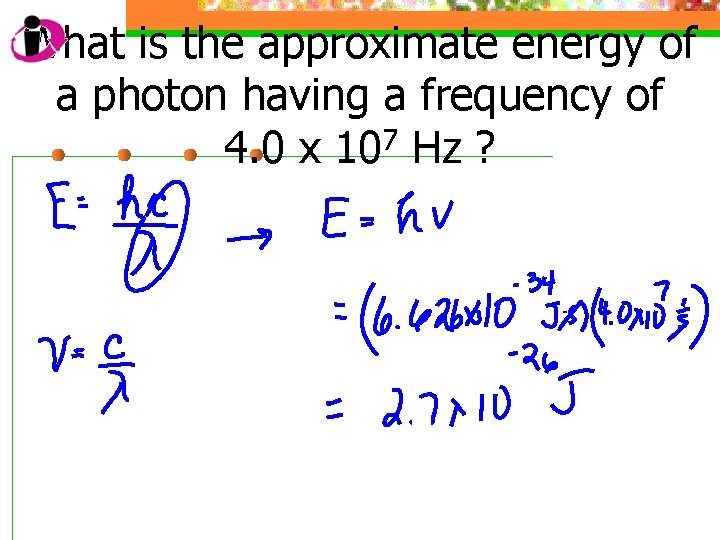
What is the approximate energy of a photon having a frequency of 4. 0 x 107 Hz ?

What is the approximate frequency of a photon having an energy of 5. 25 x 10 -24 J ?

What is the difference between a 2 s and a 3 s orbital? A) B) C) There is no difference More electrons can occupy a 3 s The 3 s is larger in volume and a greater distance from the nucleus than a 2 s

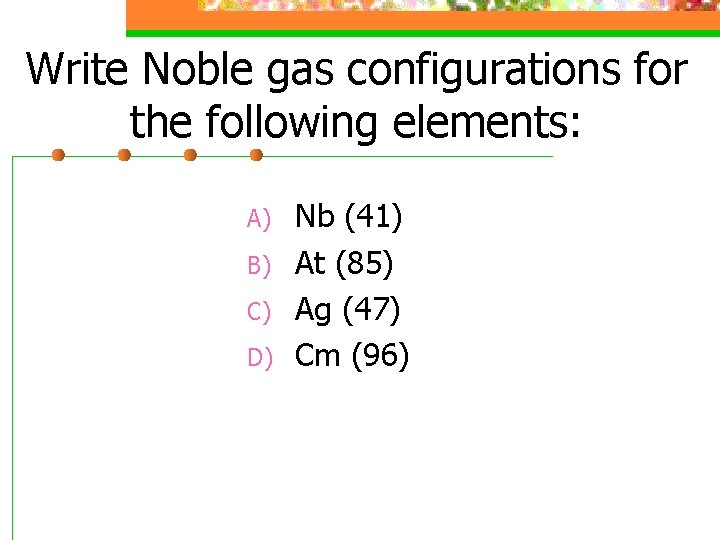
Write Noble gas configurations for the following elements: A) B) C) D) Nb (41) At (85) Ag (47) Cm (96)
![ANSWERS A B C D Nb 41 At 85 Ag 47 Cm 96 Kr ANSWERS A) B) C) D) Nb (41) At (85) Ag (47) Cm (96) [Kr]](https://slidetodoc.com/presentation_image_h/8858d7b59e81f4306f908d439e9bbb88/image-21.jpg)
ANSWERS A) B) C) D) Nb (41) At (85) Ag (47) Cm (96) [Kr] 5 s 2 4 d 3 [Xe] 6 s 2 4 f 14 5 d 10 6 p 5 [Kr] 5 s 1 4 d 10 [Rn] 7 s 2 5 f 8

What is the probability of finding an electron within the region of the electron cloud? A) B) C) D) 50% 75% 90% 100%

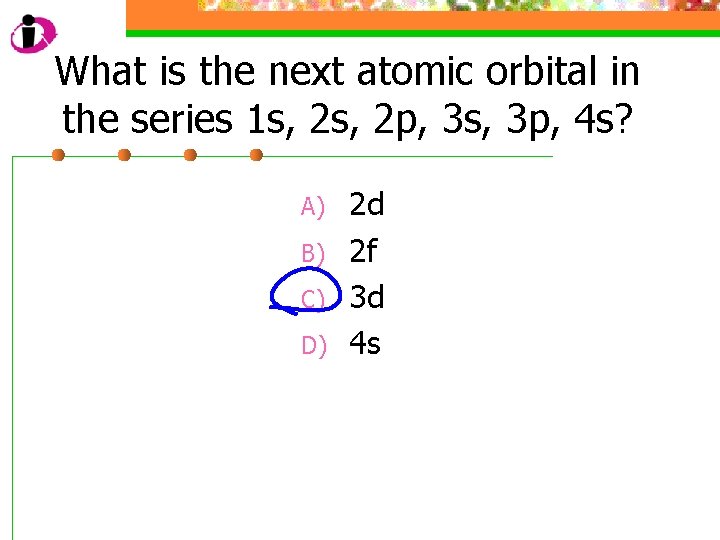
What is the next atomic orbital in the series 1 s, 2 p, 3 s, 3 p, 4 s? A) B) C) D) 2 d 2 f 3 d 4 s

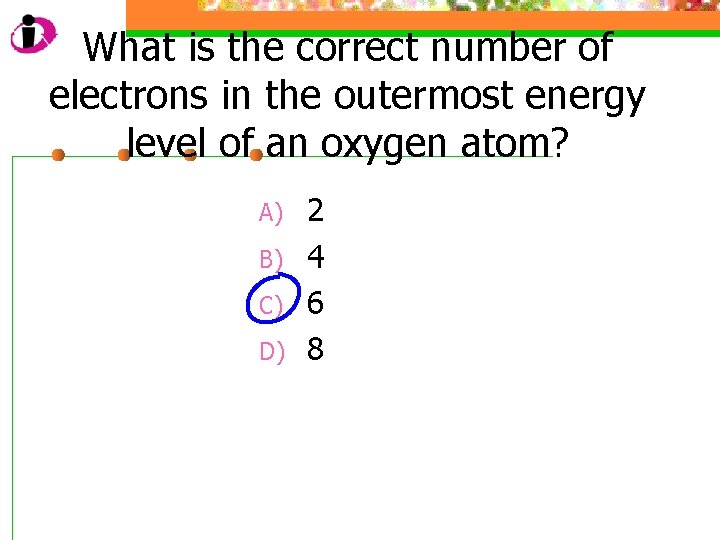
What is the correct number of electrons in the outermost energy level of an oxygen atom? A) B) C) D) 2 4 6 8

Which of the following states that no more than two electrons can occupy an atomic orbital and that two electrons in the same orbital must have opposite spins? A) B) C) D) Hund’s rule Pauli exclusion principle Dalton’s theory Aufbau principle

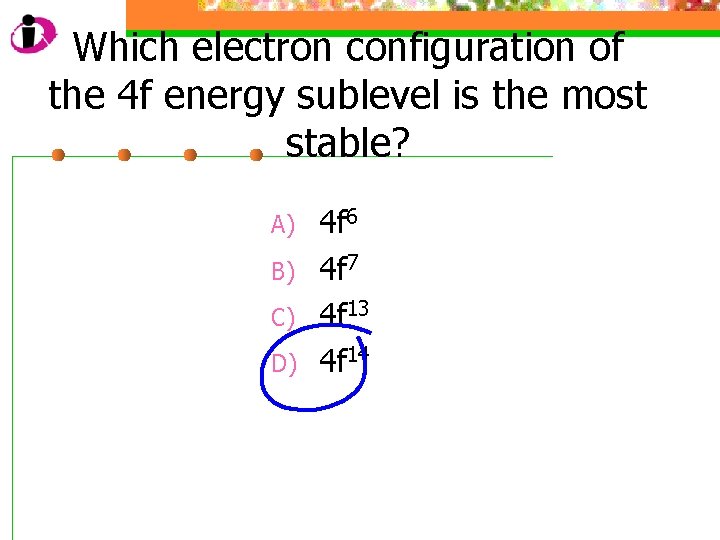
Which electron configuration of the 4 f energy sublevel is the most stable? A) B) C) D) 4 f 6 4 f 7 4 f 13 4 f 14

A wave has wavelength= -7 5. 80 x 10 m n Convert this wavelength to nanometers.

Which of the following electromagnetic waves have the highest frequencies? A) B) C) D) ultraviolet light waves X-rays Microwaves Gamma rays

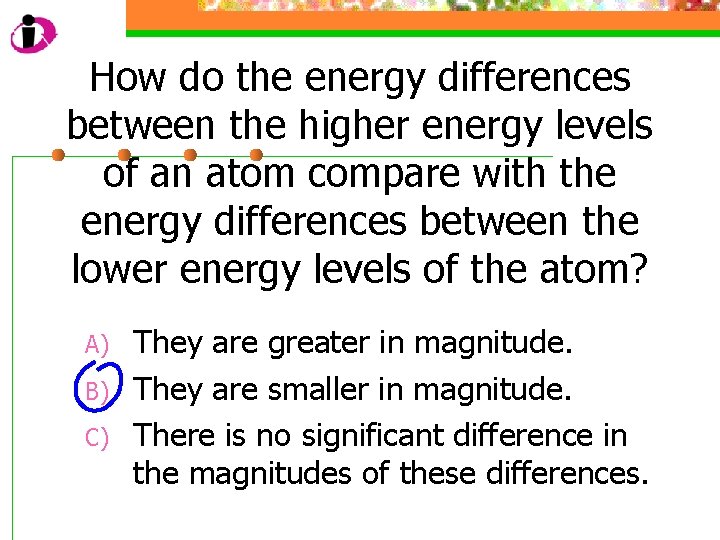
How do the energy differences between the higher energy levels of an atom compare with the energy differences between the lower energy levels of the atom? A) B) C) They are greater in magnitude. They are smaller in magnitude. There is no significant difference in the magnitudes of these differences.

Things to know……. . 1. 2. 3. 4. Principles of electron configurations Explanation for discrete lines in atomic emission spectra You will be given c and h as well as a periodic table. Hydrogen’s spectrum and series of lines

Suggested Book Problems n Pg. 149 22, 25, 27, 29, 30, 31, 33, 35, 37, 39, 40, 41, 44, 49, 50, 55, 58, 59, 61, 63, 65, 68