Clicker Questions Chapter 2 Atoms and Elements Allison














- Slides: 37

Clicker Questions Chapter 2 Atoms and Elements Allison Soult University of Kentucky © 2014 Pearson Education, Inc.

Two samples of the same compound are compared: Sample 1: 24. 22 g carbon and 32. 00 g oxygen Sample 2: 36. 22 g carbon and 48. 00 g oxygen This data shows which of the following? a) b) c) d) e) The law of definite proportions The law of multiple proportions The law of conservation of mass a and b only a, b, and c © 2014 Pearson Education, Inc.

Two samples of the same compound are compared: Sample 1: 24. 22 g carbon and 32. 00 g oxygen Sample 2: 36. 22 g carbon and 48. 00 g oxygen This data shows which of the following? a) b) c) d) e) The law of definite proportions The law of multiple proportions The law of conservation of mass a and b only a, b, and c © 2014 Pearson Education, Inc.

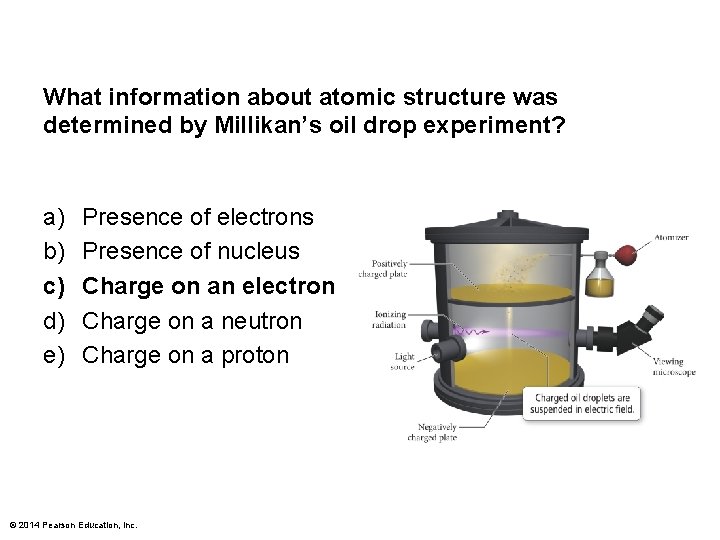
What information about atomic structure was determined by Millikan’s oil drop experiment? a) b) c) d) e) Presence of electrons Presence of nucleus Charge on an electron Charge on a neutron Charge on a proton © 2014 Pearson Education, Inc.

What information about atomic structure was determined by Millikan’s oil drop experiment? a) b) c) d) e) Presence of electrons Presence of nucleus Charge on an electron Charge on a neutron Charge on a proton © 2014 Pearson Education, Inc.

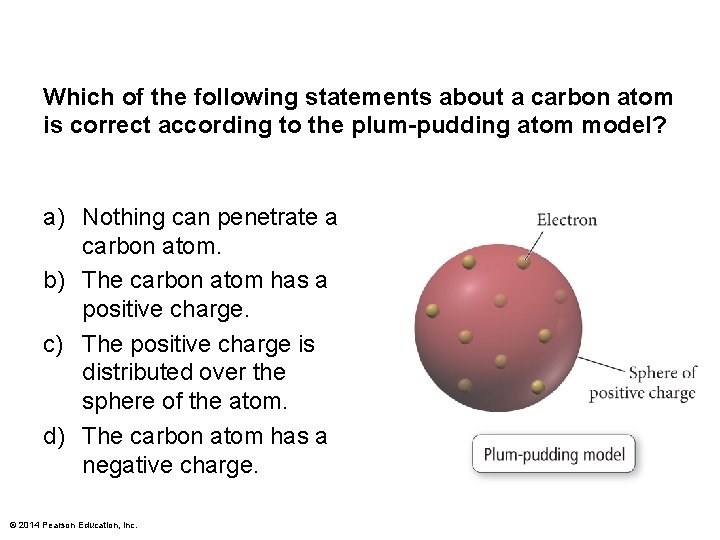
Which of the following statements about a carbon atom is correct according to the plum-pudding atom model? a) Nothing can penetrate a carbon atom. b) The carbon atom has a positive charge. c) The positive charge is distributed over the sphere of the atom. d) The carbon atom has a negative charge. © 2014 Pearson Education, Inc.

Which of the following statements about a carbon atom is correct according to the plum-pudding atom model? a) Nothing can penetrate a carbon atom. b) The carbon atom has a positive charge. c) The positive charge is distributed over the sphere of the atom. d) The carbon atom has a negative charge. © 2014 Pearson Education, Inc.

Which scientist confirmed that most of an atom’s mass and all of its positive charge are contained in a small core called the nucleus? a) b) c) d) e) John Dalton Joseph Proust J. J. Thomson Ernest Rutherford Robert Milliakn © 2014 Pearson Education, Inc.

Which scientist confirmed that most of an atom’s mass and all of its positive charge are contained in a small core called the nucleus? a) b) c) d) e) John Dalton Joseph Proust J. J. Thomson Ernest Rutherford Robert Milliakn © 2014 Pearson Education, Inc.

Which of the following determines the identity of an atom? a) b) c) d) e) Number of protons Number of electrons Number of neutrons Total number of protons and electrons © 2014 Pearson Education, Inc.

Which of the following determines the identity of an atom? a) b) c) d) e) Number of protons Number of electrons Number of neutrons Total number of protons and electrons © 2014 Pearson Education, Inc.

How many neutrons are in 1000 atoms of Cl-37? a) b) c) d) e) 37 37, 000 20 20, 000 Cannot be determined © 2014 Pearson Education, Inc.

How many neutrons are in 1000 atoms of Cl-37? a) b) c) d) e) 37 37, 000 20 20, 000 Cannot be determined © 2014 Pearson Education, Inc.

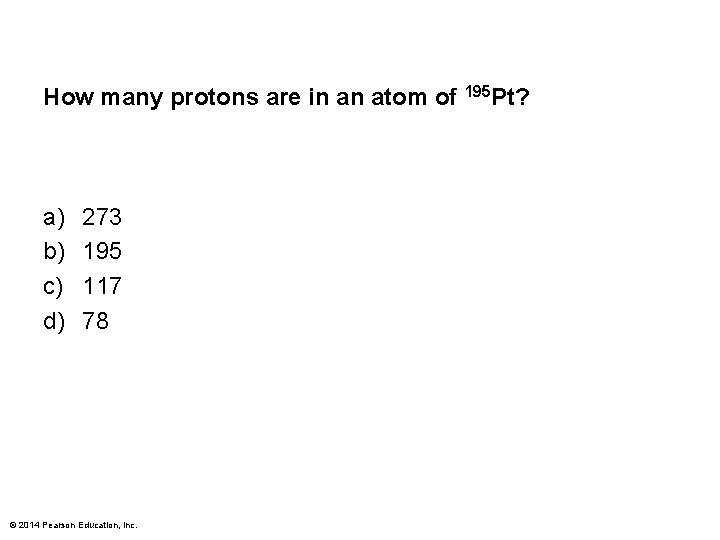
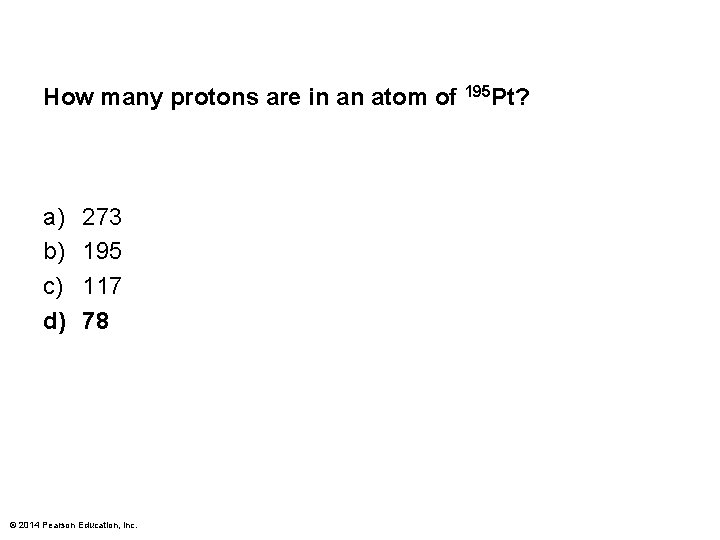
How many protons are in an atom of 195 Pt? a) b) c) d) 273 195 117 78 © 2014 Pearson Education, Inc.

How many protons are in an atom of 195 Pt? a) b) c) d) 273 195 117 78 © 2014 Pearson Education, Inc.

Which of the following statements is true about ions? a) An ion is the result of an atom gaining a neutron. b) A positively charged ion results from the gain of a proton. c) A negatively charged ion results from the loss of a proton. d) The gain or loss of an electron by an atom will result in an ion. e) None of the above. © 2014 Pearson Education, Inc.

Which of the following statements is true about ions? a) An ion is the result of an atom gaining a neutron. b) A positively charged ion results from the gain of a proton. c) A negatively charged ion results from the loss of a proton. d) The gain or loss of an electron by an atom will result in an ion. e) None of the above. © 2014 Pearson Education, Inc.

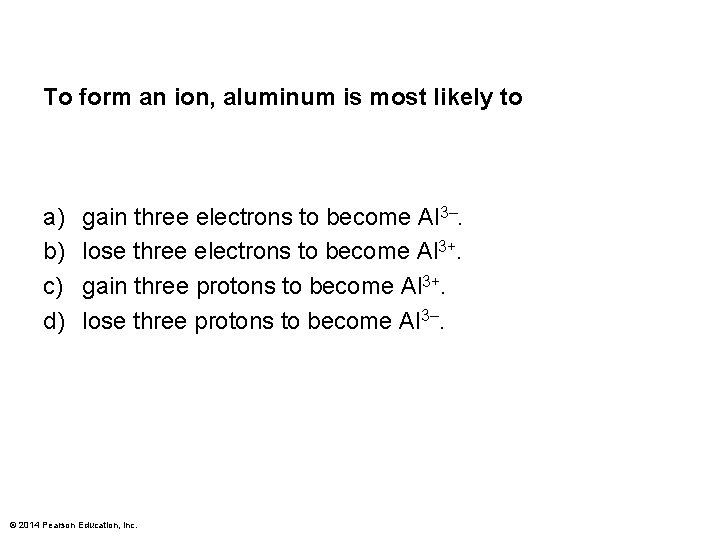
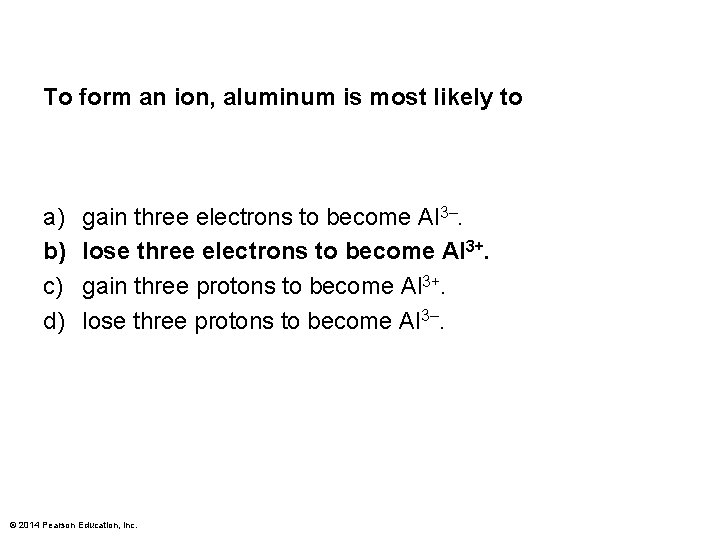
To form an ion, aluminum is most likely to a) b) c) d) gain three electrons to become Al 3–. lose three electrons to become Al 3+. gain three protons to become Al 3+. lose three protons to become Al 3–. © 2014 Pearson Education, Inc.

To form an ion, aluminum is most likely to a) b) c) d) gain three electrons to become Al 3–. lose three electrons to become Al 3+. gain three protons to become Al 3+. lose three protons to become Al 3–. © 2014 Pearson Education, Inc.

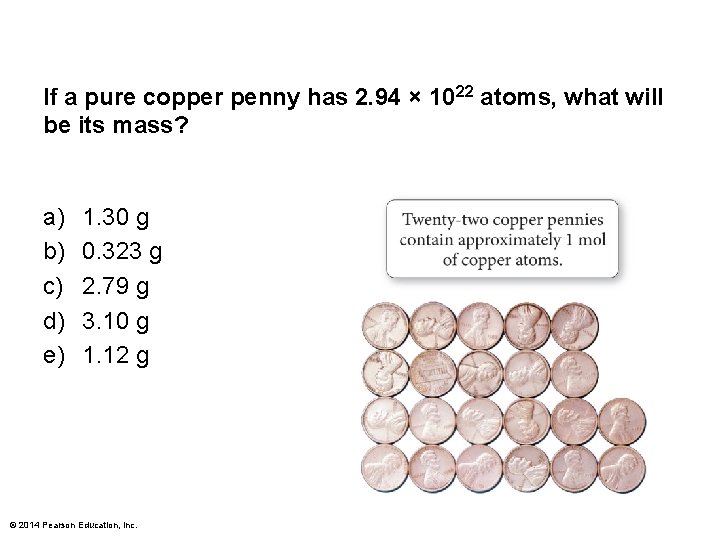
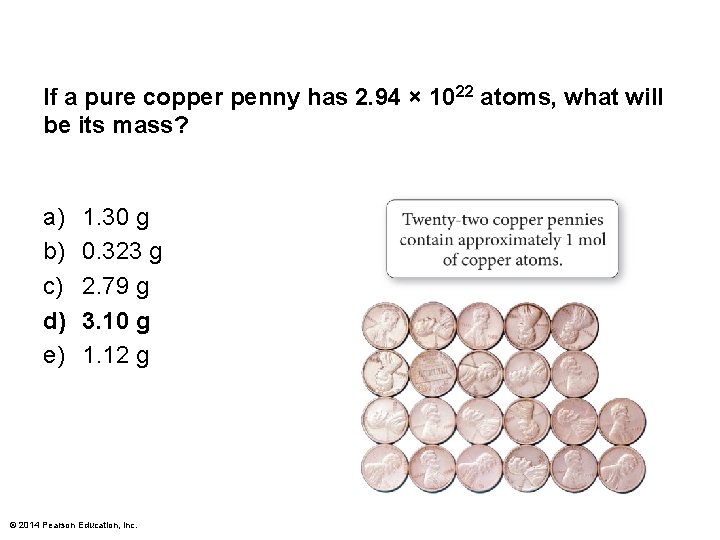
If a pure copper penny has 2. 94 × 1022 atoms, what will be its mass? a) b) c) d) e) 1. 30 g 0. 323 g 2. 79 g 3. 10 g 1. 12 g © 2014 Pearson Education, Inc.

If a pure copper penny has 2. 94 × 1022 atoms, what will be its mass? a) b) c) d) e) 1. 30 g 0. 323 g 2. 79 g 3. 10 g 1. 12 g © 2014 Pearson Education, Inc.

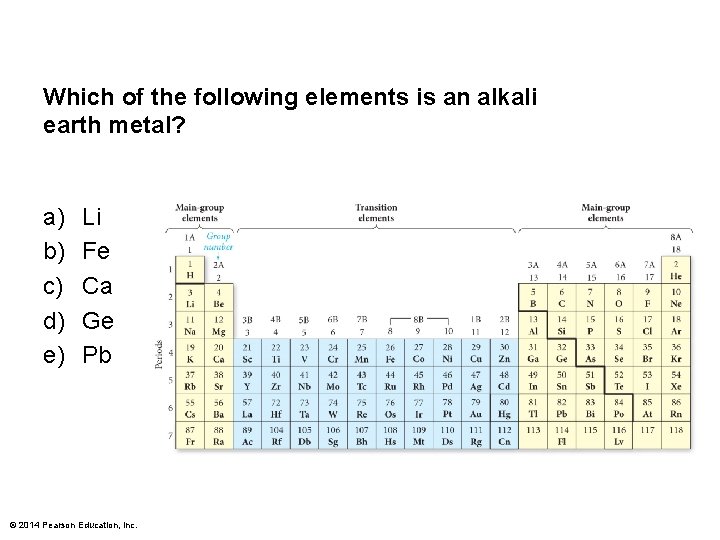
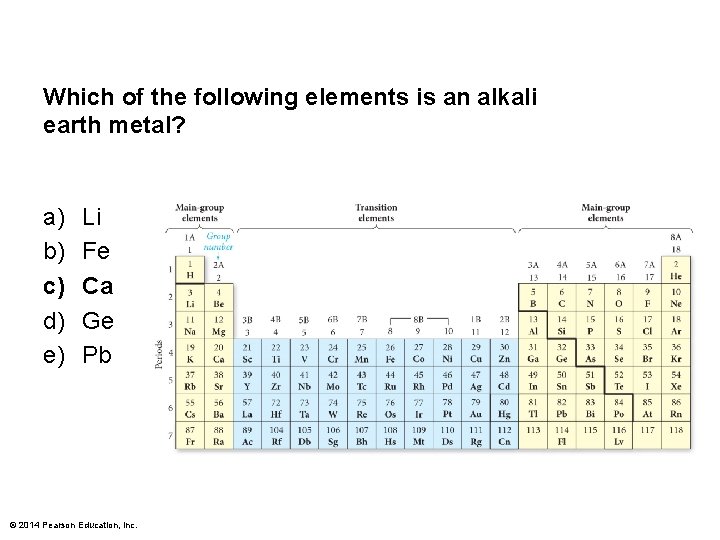
Which of the following elements is an alkali earth metal? a) b) c) d) e) Li Fe Ca Ge Pb © 2014 Pearson Education, Inc.

Which of the following elements is an alkali earth metal? a) b) c) d) e) Li Fe Ca Ge Pb © 2014 Pearson Education, Inc.

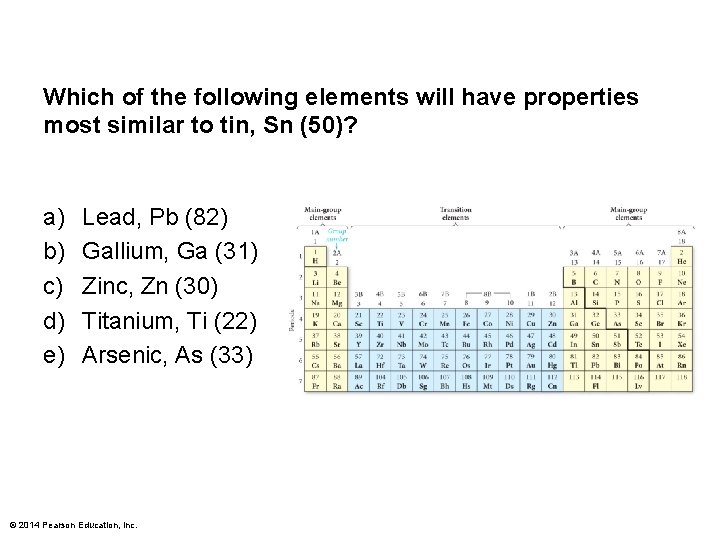
Which of the following elements will have properties most similar to tin, Sn (50)? a) b) c) d) e) Lead, Pb (82) Gallium, Ga (31) Zinc, Zn (30) Titanium, Ti (22) Arsenic, As (33) © 2014 Pearson Education, Inc.

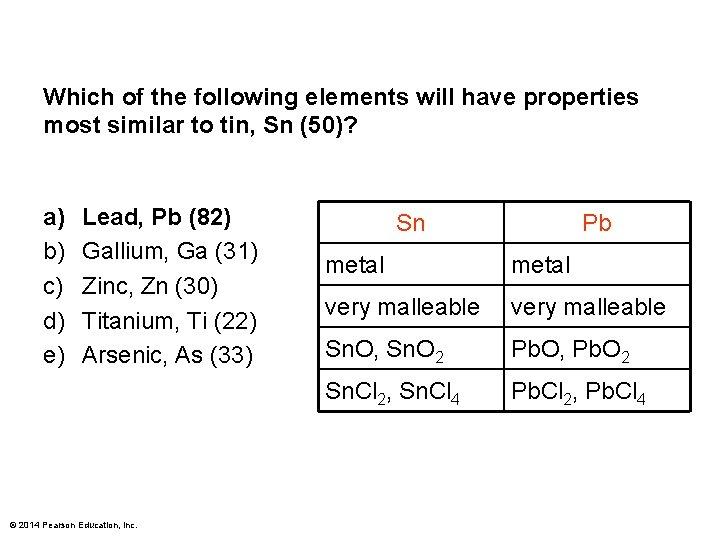
Which of the following elements will have properties most similar to tin, Sn (50)? a) b) c) d) e) Lead, Pb (82) Gallium, Ga (31) Zinc, Zn (30) Titanium, Ti (22) Arsenic, As (33) © 2014 Pearson Education, Inc. Sn Pb metal very malleable Sn. O, Sn. O 2 Pb. O, Pb. O 2 Sn. Cl 2, Sn. Cl 4 Pb. Cl 2, Pb. Cl 4

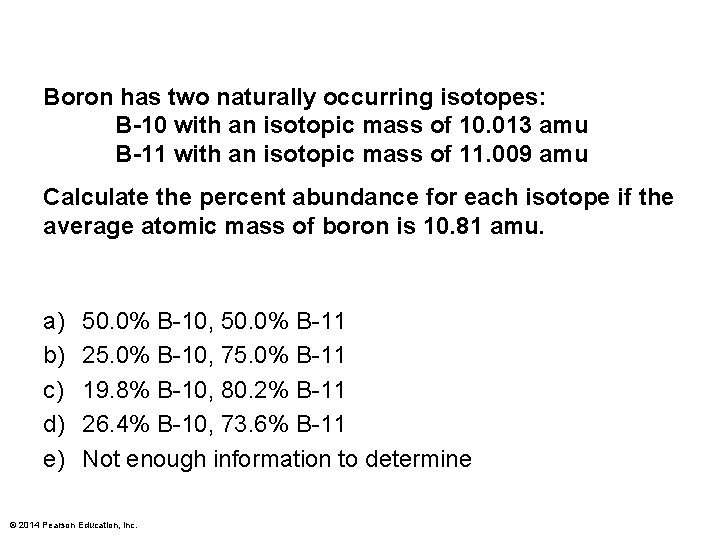
Boron has two naturally occurring isotopes: B-10 with an isotopic mass of 10. 013 amu B-11 with an isotopic mass of 11. 009 amu Calculate the percent abundance for each isotope if the average atomic mass of boron is 10. 81 amu. a) b) c) d) e) 50. 0% B-10, 50. 0% B-11 25. 0% B-10, 75. 0% B-11 19. 8% B-10, 80. 2% B-11 26. 4% B-10, 73. 6% B-11 Not enough information to determine © 2014 Pearson Education, Inc.

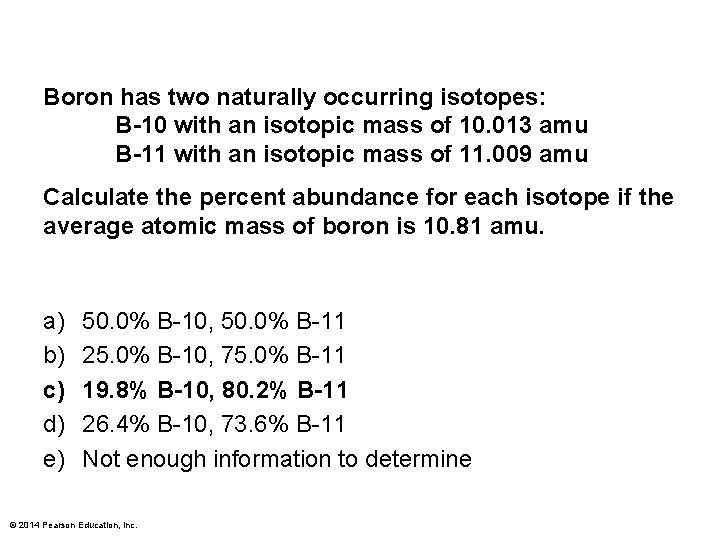
Boron has two naturally occurring isotopes: B-10 with an isotopic mass of 10. 013 amu B-11 with an isotopic mass of 11. 009 amu Calculate the percent abundance for each isotope if the average atomic mass of boron is 10. 81 amu. a) b) c) d) e) 50. 0% B-10, 50. 0% B-11 25. 0% B-10, 75. 0% B-11 19. 8% B-10, 80. 2% B-11 26. 4% B-10, 73. 6% B-11 Not enough information to determine © 2014 Pearson Education, Inc.

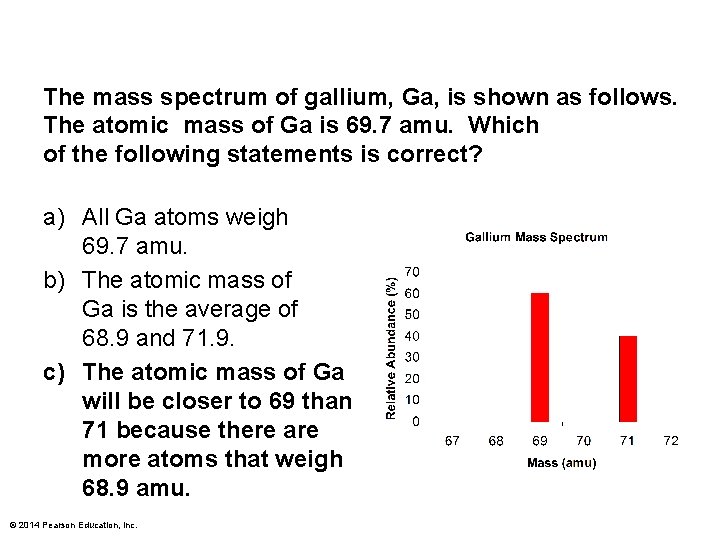
The mass spectrum of gallium, Ga, is shown as follows. The atomic mass of Ga is 69. 7 amu. Which of the following statements is correct? a) All Ga atoms weigh 69. 7 amu. b) The atomic mass of Ga is the average of 68. 9 and 71. 9. c) The atomic mass of Ga will be closer to 69 than 71 because there are more atoms that weigh 68. 9 amu. © 2014 Pearson Education, Inc.

The mass spectrum of gallium, Ga, is shown as follows. The atomic mass of Ga is 69. 7 amu. Which of the following statements is correct? a) All Ga atoms weigh 69. 7 amu. b) The atomic mass of Ga is the average of 68. 9 and 71. 9. c) The atomic mass of Ga will be closer to 69 than 71 because there are more atoms that weigh 68. 9 amu. © 2014 Pearson Education, Inc.

Which of the following is FALSE? a) The mole can be used to specify Avogadro’s number of anything. b) Avogadro’s number, 6. 022 × 1023, is an exact number. c) The mole is equal to the number of atoms in exactly 12 grams of carbon-12. d) The value of an element’s molar mass in grams per mole is numerically equal to the element’s atomic mass in amu. © 2014 Pearson Education, Inc.

Which of the following is FALSE? a) The mole can be used to specify Avogadro’s number of anything. b) Avogadro’s number, 6. 022 × 1023, is an exact number. c) The mole is equal to the number of atoms in exactly 12 grams of carbon-12. d) The value of an element’s molar mass in grams per mole is numerically equal to the element’s atomic mass in amu. © 2014 Pearson Education, Inc.

Which has more atoms, 10. 0 g Mg or 10. 0 g Ca? a) Magnesium b) Calcium c) Both have the same number of atoms. © 2014 Pearson Education, Inc.

Which has more atoms, 10. 0 g Mg or 10. 0 g Ca? a) Magnesium b) Calcium c) Both have the same number of atoms. © 2014 Pearson Education, Inc.

Calculate the mass in grams of one mole of footballs if one football has a mass of 0. 43 kg. a) b) c) d) e) 430 g 1. 4 g 2. 6 × 1026 g 7. 1 × 10– 23 g 1. 4 × 1027 g © 2014 Pearson Education, Inc.

Calculate the mass in grams of one mole of footballs if one football has a mass of 0. 43 kg. a) b) c) d) e) 430 g 1. 4 g 2. 6 × 1026 g 7. 1 × 10– 23 g 1. 4 × 1027 g © 2014 Pearson Education, Inc.

Which of the following has the largest mass? a) b) c) d) e) 10. 0 g Li 10. 0 moles of Li 100 g Na 10. 0 moles of K 100 g Rb © 2014 Pearson Education, Inc.

Which of the following has the largest mass? a) b) c) d) e) 10. 0 g Li 10. 0 moles of Li 100 g Na 10. 0 moles of K 100 g Rb © 2014 Pearson Education, Inc.