1 14 6 2 18 10 15 7













- Slides: 21

1 14 6 2 18 10 15 7 3 19 11 16 8 4 17 9 12 20 WHACK-A-MOLE 13 5

1 How many electrons are in an neutral atom of sodium? Students type their answers here

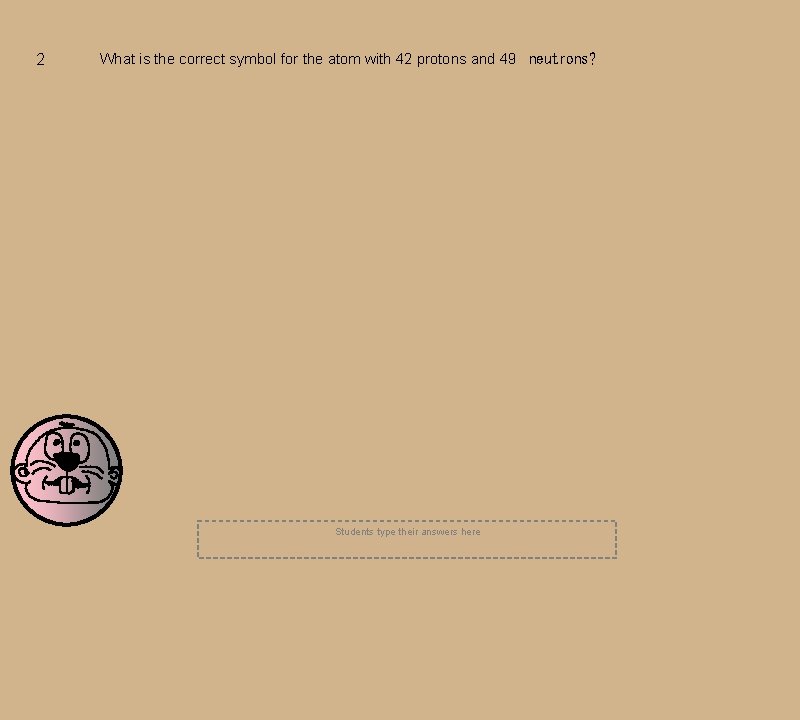
2 What is the correct symbol for the atom with 42 protons and 49 neutrons? Students type their answers here

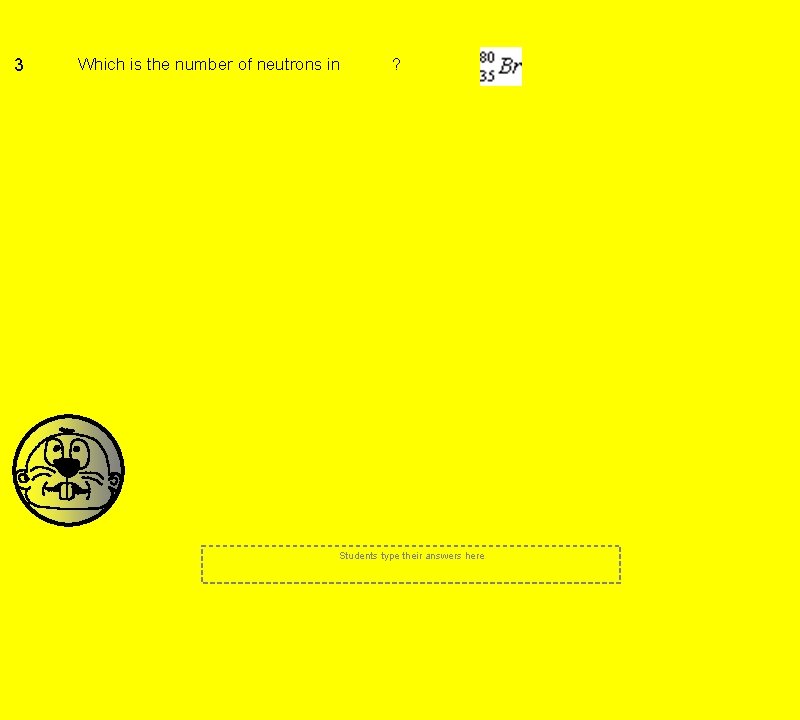
3 Which is the number of neutrons in ? Students type their answers here

4 What element has 14 protons? Students type their answers here

5 An atom has 23 protons and 29 neutrons. Which is the correct chemical symbol for this atom? Students type their answers here

6 An industrially important element contains 26 electrons and rusts in the presence of air and moisture. Identify the element. Students type their answers here

7 What has a charge of +1? Students type their answers here

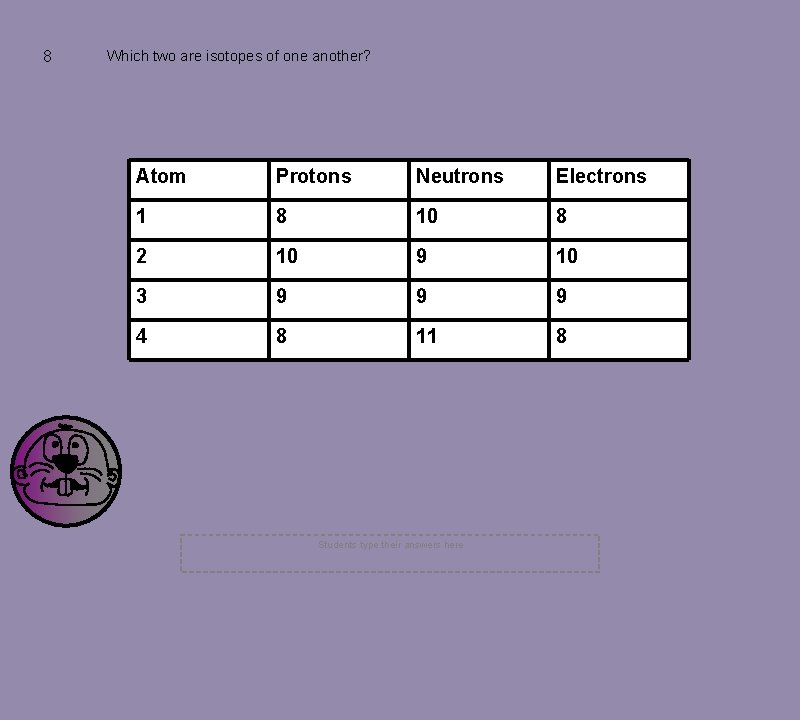
8 Which two are isotopes of one another? Atom Protons Neutrons Electrons 1 8 10 8 2 10 9 10 3 9 9 9 4 8 11 8 Students type their answers here

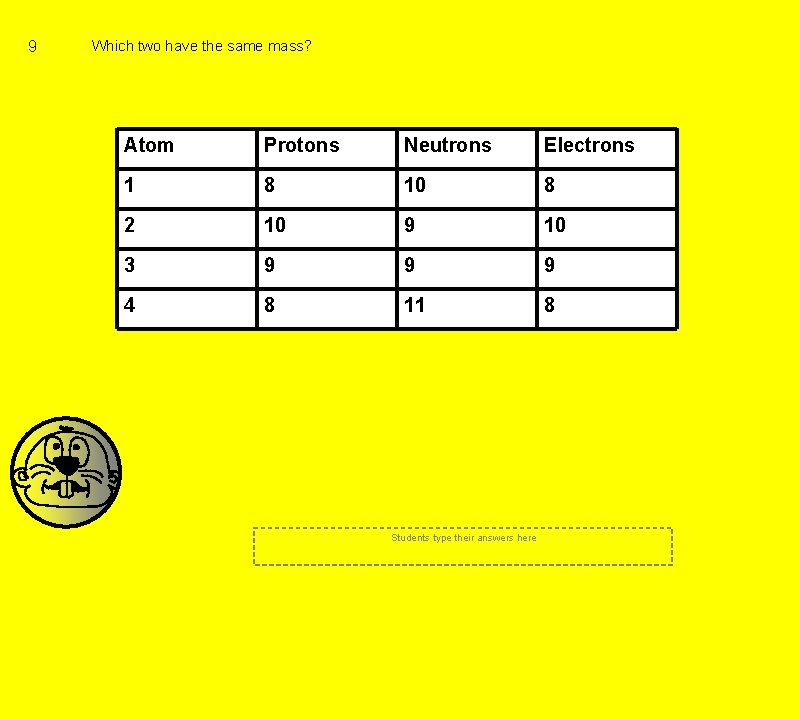
9 Which two have the same mass? Atom Protons Neutrons Electrons 1 8 10 8 2 10 9 10 3 9 9 9 4 8 11 8 Students type their answers here

10 Which is the smallest part of an element that retains all the properties of the element? Students type their answers here

11 How many neutrons are in an atom of chlorine? Students type their answers here

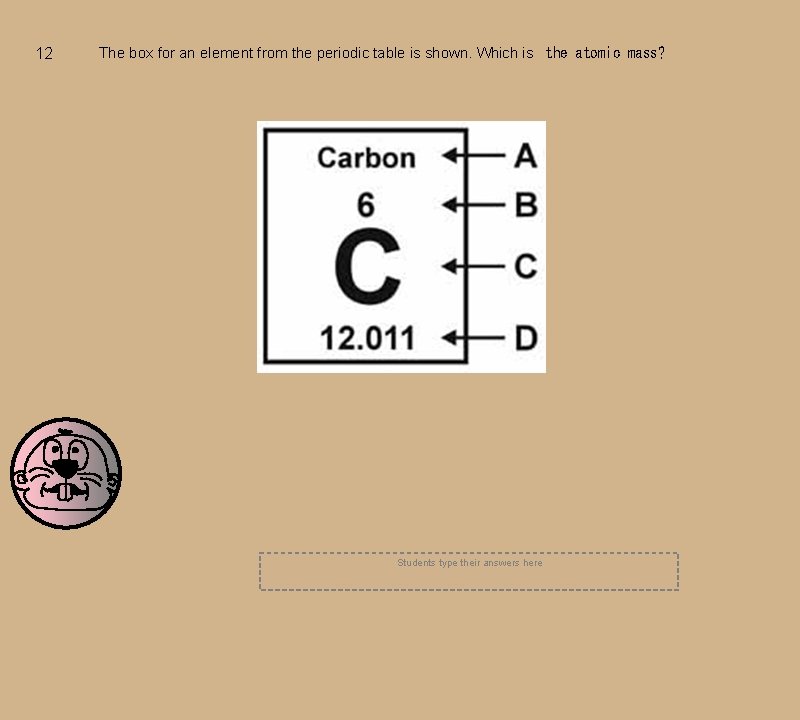
12 The box for an element from the periodic table is shown. Which is the atomic mass? Students type their answers here

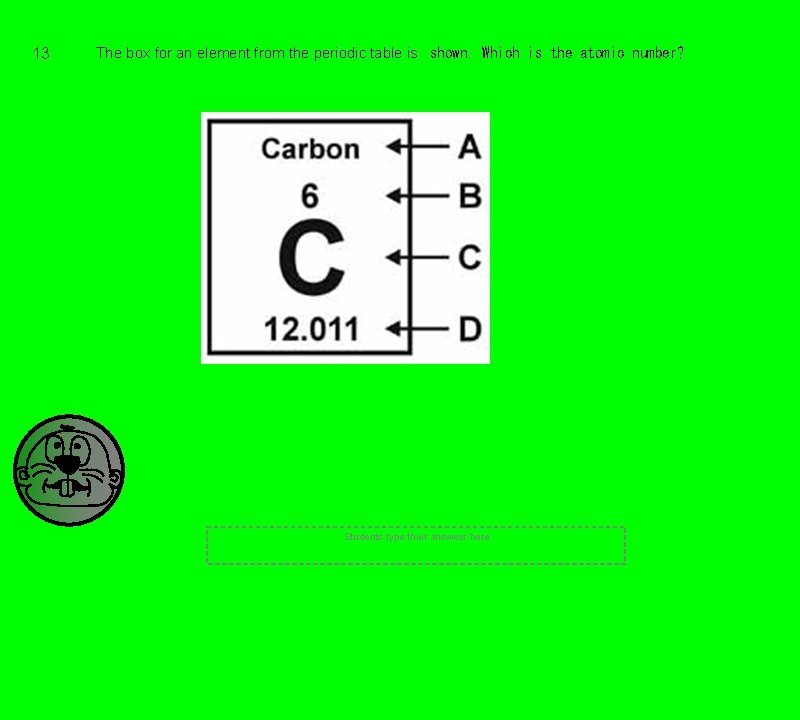
13 The box for an element from the periodic table is shown. Which is the atomic number? Students type their answers here

14 Which scientist described a positively charged core (“nucleus”) in the middle of a lot of empty space? Students type their answers here

15 Which scientist described an atom made of a solid positively charged substance with electrons disperse throughout it? Students type their answers here

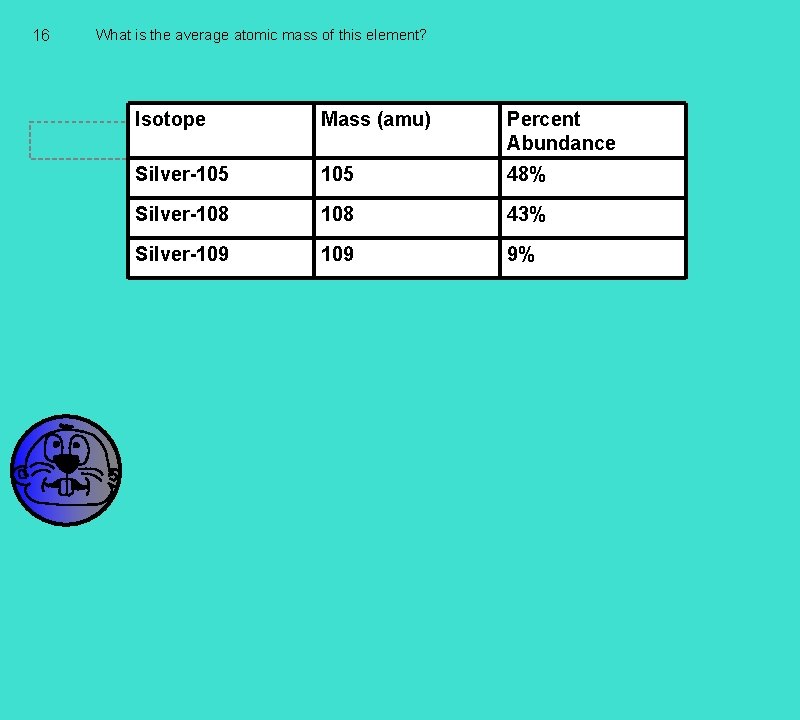
16 What is the average atomic mass of this element? Isotope Mass (amu) Students type their answers here Percent Abundance Silver-105 48% Silver-108 43% Silver-109 9%

17 Write the chemical symbol for the isotope of cobalt with 37 neutrons. Students type their answers here

18 Identify the missing particle in the equation below. Justify your response. Students type their answers here

19 Identify the element containing 34 protons. Students type their answers here

20 Fast moving electrons travel through the empty space surrounding the nucleus. How are electrons held within the atom? Students type their answers here