Daily Review Which scientist discovered the electron Which






- Slides: 14

Daily Review • • • Which scientist discovered the electron? Which scientist discovered the nucleus? Which scientist had the plum pudding model of the atom? Which scientist used the gold foil experiment? Which scientist described electrons as having set paths? Which scientist believed that atoms were indivisible?

Atomic Structure Chapter 4

Objectives • Identify the subatomic particles and understand placement and charge for each particle. • Understand the concept of an isotope and what makes one element different from another • Calculate the number of neutrons in an atom • Calculate the Average atomic mass of a isotope

The Atom • Matter is anything that takes up space and has mass. All matter is made of atoms. • Atoms are the basic building blocks of matter. They make up everything around us; Your desk, the board, your body, everything is made of atoms! • Atoms are too small to see without powerful microscopes.

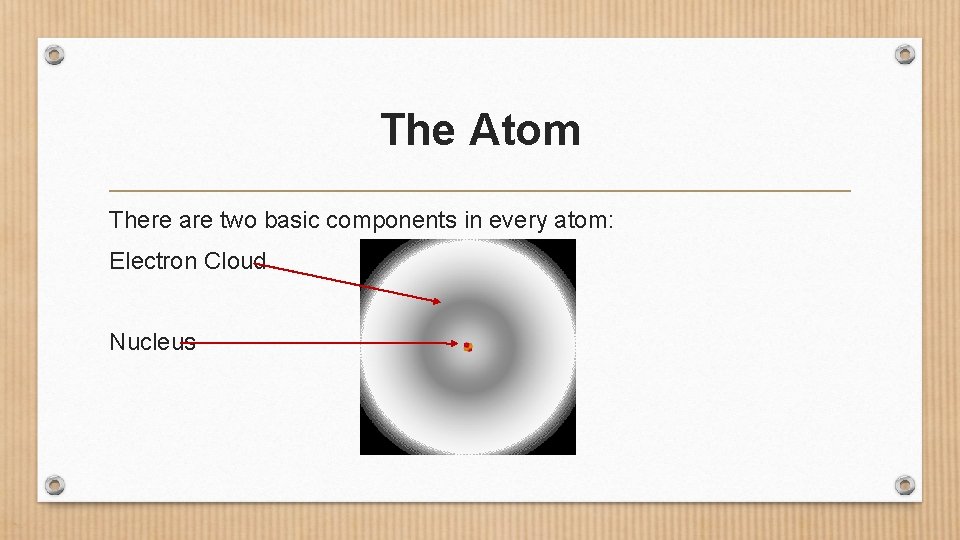
The Atom There are two basic components in every atom: Electron Cloud Nucleus

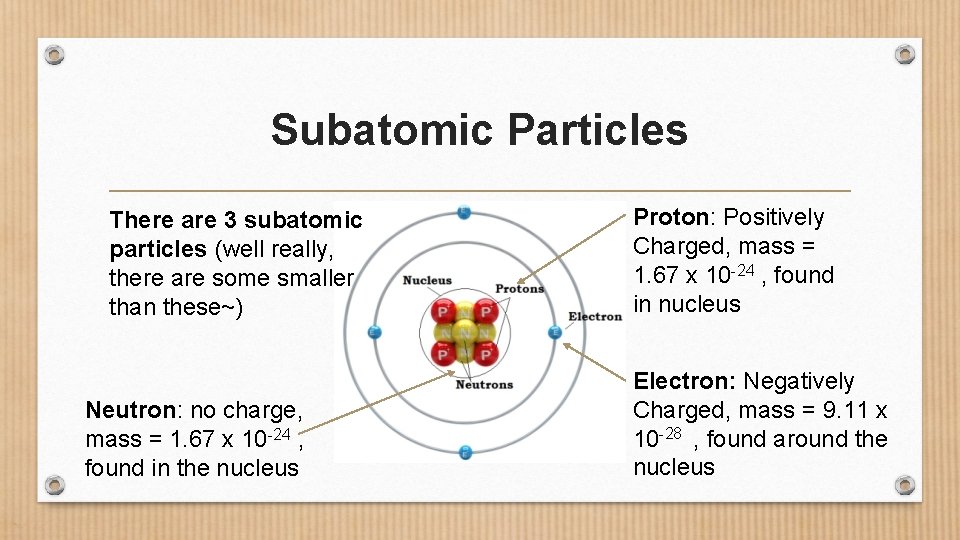
Subatomic Particles There are 3 subatomic particles (well really, there are some smaller than these~) Neutron: no charge, mass = 1. 67 x 10 -24 , found in the nucleus Proton: Positively Charged, mass = 1. 67 x 10 -24 , found in nucleus Electron: Negatively Charged, mass = 9. 11 x 10 -28 , found around the nucleus

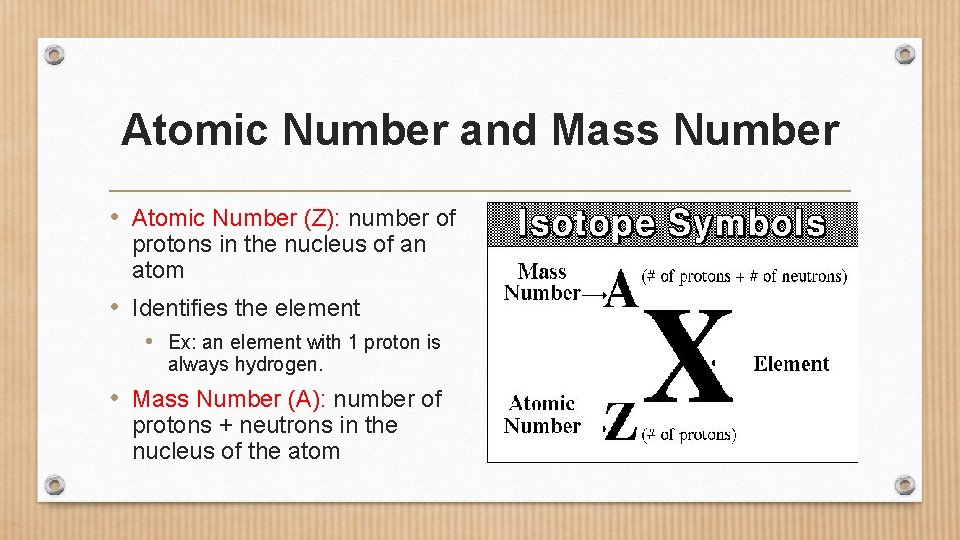
Atomic Number and Mass Number • Atomic Number (Z): number of protons in the nucleus of an atom • Identifies the element • Ex: an element with 1 proton is always hydrogen. • Mass Number (A): number of protons + neutrons in the nucleus of the atom

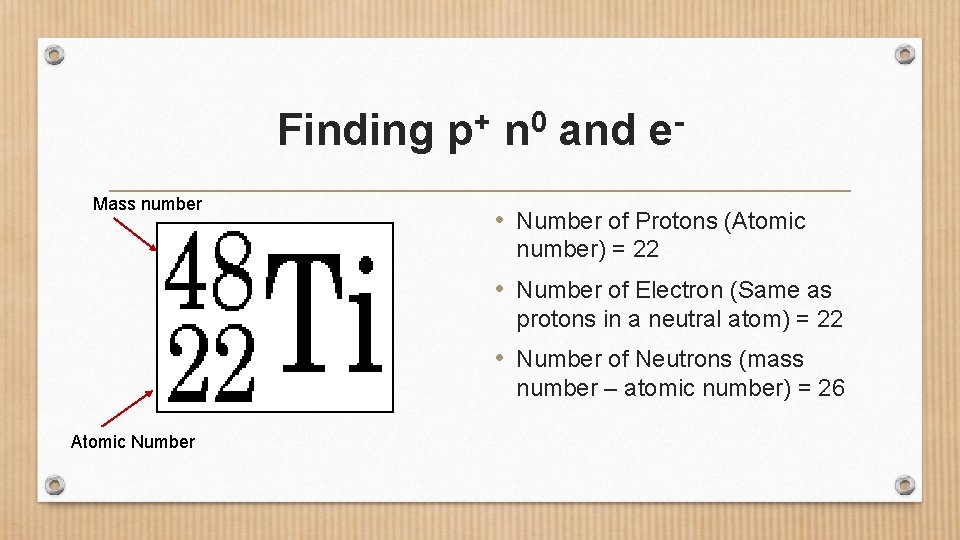
Finding Mass number + p 0 n and e • Number of Protons (Atomic number) = 22 • Number of Electron (Same as protons in a neutral atom) = 22 • Number of Neutrons (mass number – atomic number) = 26 Atomic Number

Finding + p Mass number Atomic Number Protons 23 11 27 13 128 53 108 47 0 n and Neutrons e Electrons

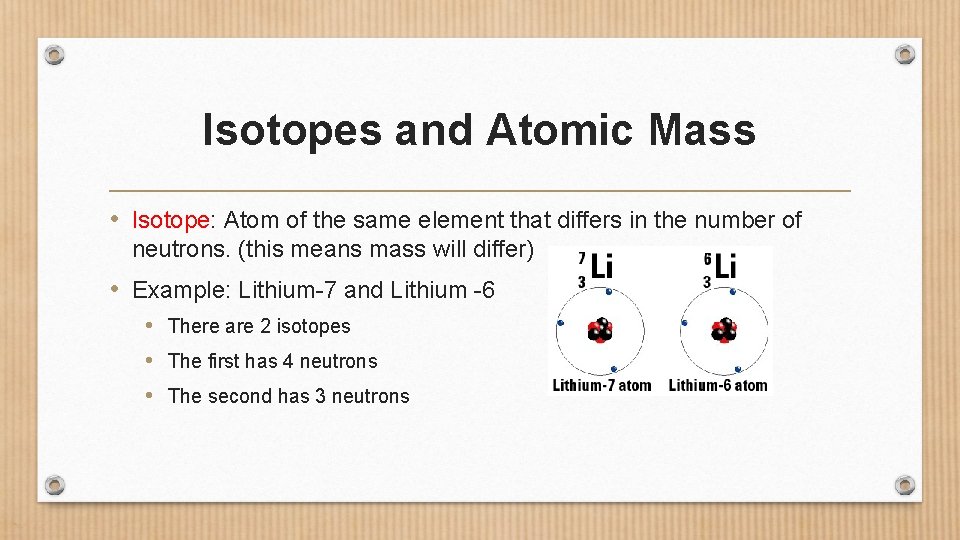
Isotopes and Atomic Mass • Isotope: Atom of the same element that differs in the number of neutrons. (this means mass will differ) • Example: Lithium-7 and Lithium -6 • There are 2 isotopes • The first has 4 neutrons • The second has 3 neutrons

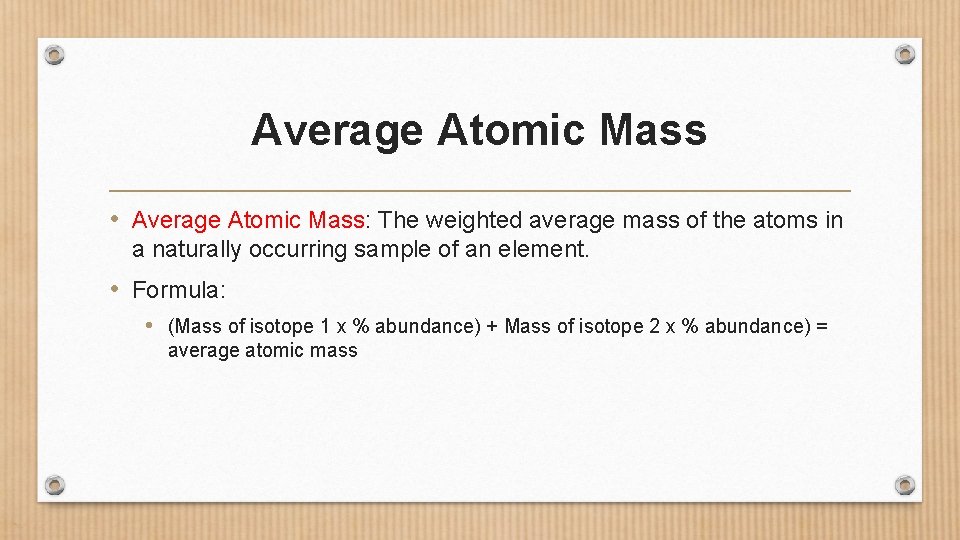
Average Atomic Mass • Average Atomic Mass: The weighted average mass of the atoms in a naturally occurring sample of an element. • Formula: • (Mass of isotope 1 x % abundance) + Mass of isotope 2 x % abundance) = average atomic mass

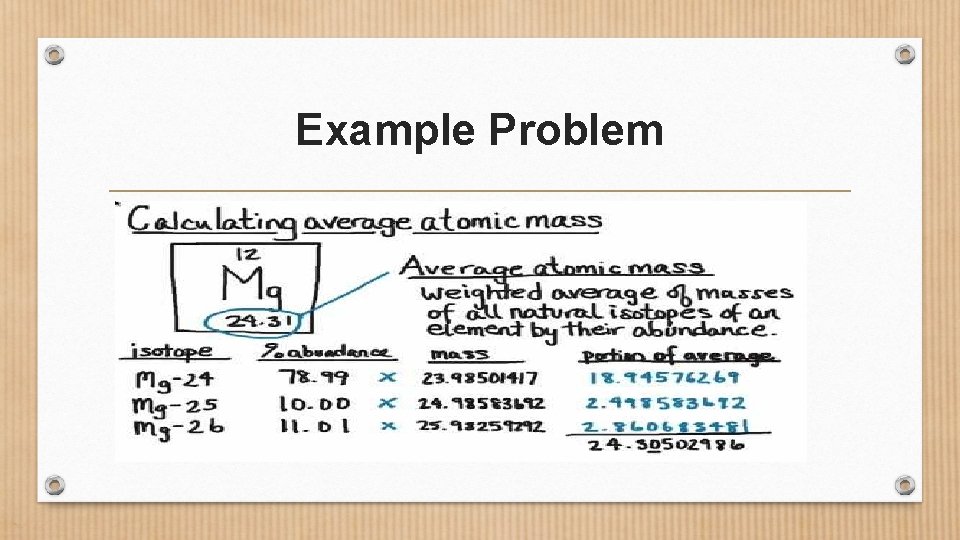
Example Problem

Practice, Practice! • Find the average atomic mass of carbon if carbon— 12 has a % abundance of 98. 89 and carbon— 13 has a percent abundance of 1. 11. • Find the average atomic mass of element X if isotope 1 has a mass of 10. 012 amu (% abundance 19. 91) and isotope 2 has a mass of 11. 009 amu (% abundance 80. 09)

Closing: K-L-W On an index card list your KLW for the lesson Know (previous knowledge) Want to know (clarification) Learned