Atoms very small If a helium atom was







- Slides: 16

Atoms: very small If a helium atom was the size of a full stop, then the average gerbil would be the size of the Earth.

Atoms: very small Now let’s pretend that the helium atom on the right is the size of the Earth. What’s wrong with this simple picture?

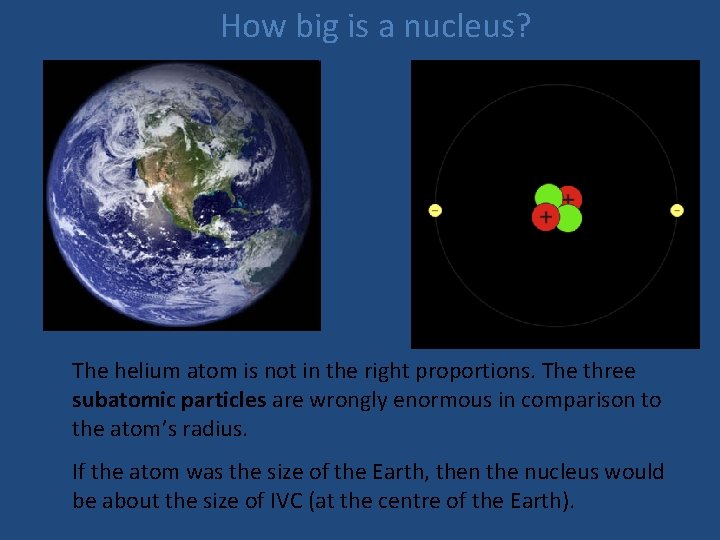
How big is a nucleus? The helium atom is not in the right proportions. The three subatomic particles are wrongly enormous in comparison to the atom’s radius. If the atom was the size of the Earth, then the nucleus would be about the size of IVC (at the centre of the Earth).

Most of the atom is empty space! If you imagine an atom being the size of Wembley stadium, the nucleus would be about the size of a football on the centre spot. The electrons would be two peas flying around the whole stadium. The rest of it: emptiness.

Atoms • Atoms are incredibly tiny. It is not possible to see an individual atom, let alone see what is inside one. • Atoms are the smallest particles of an element that can take part in chemical reactions. To understand their structure, scientists have concluded an even smaller object at its centre, called a nucleus. • This is about 20 000 times smaller than the atom itself.



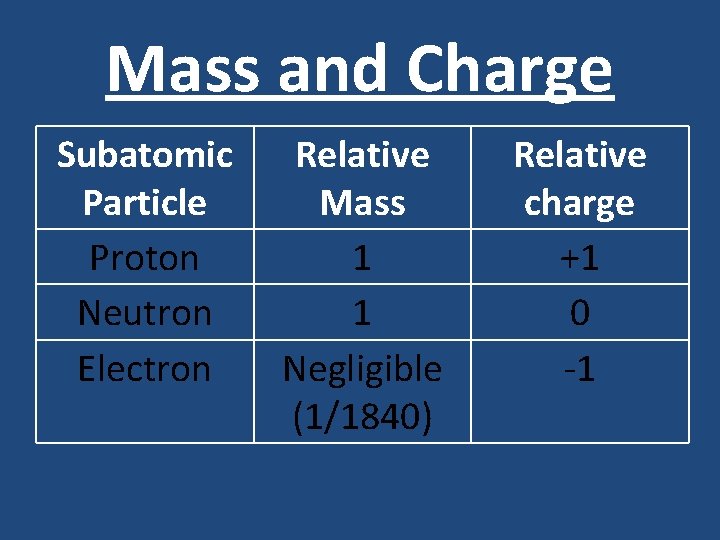
Subatomic Particles • Atoms are made from three types of subatomic particles. The nucleus contains protons and neutrons. • It is surrounded by electrons. The electrons are arranged in shells (or energy levels) at different distances from the nucleus • Subatomic particles have very, very small masses and electrical charges. It is more convenient to describe their masses can charges compared to a proton. • These are called the particles relative mass and relative charge

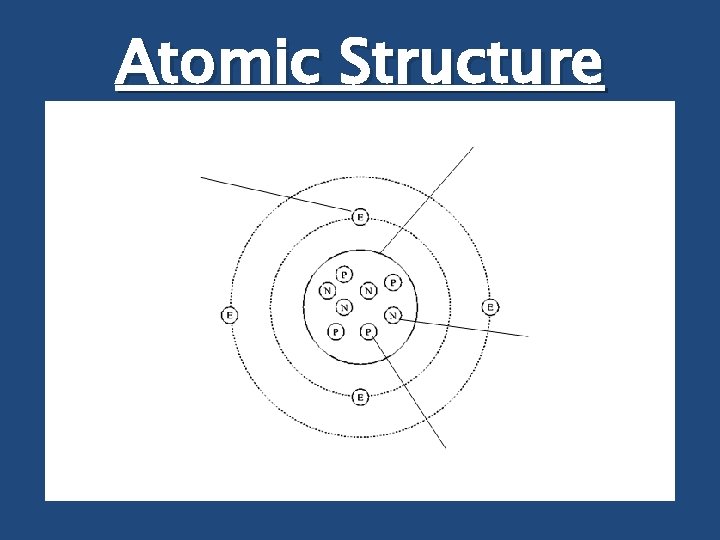
Atomic Structure Electron Nucleus Neutron Proton

Mass and Charge Subatomic Particle Proton Neutron Electron Relative Mass 1 1 Negligible (1/1840) Relative charge +1 0 -1

Atoms and Elements • Every atom of a particular element has the same number of protons in their atoms. • For example, all hydrogen atoms contain one proton, all helium atoms contain two protons. • This means that they have no overall charge.

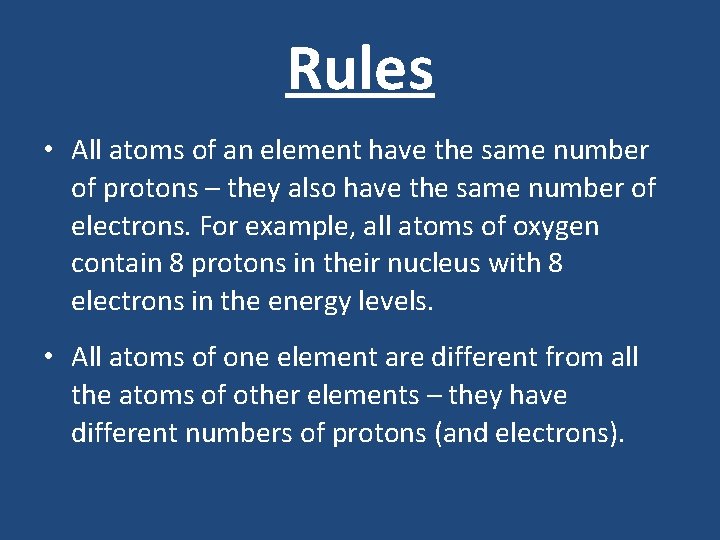
Rules • All atoms of an element have the same number of protons – they also have the same number of electrons. For example, all atoms of oxygen contain 8 protons in their nucleus with 8 electrons in the energy levels. • All atoms of one element are different from all the atoms of other elements – they have different numbers of protons (and electrons).

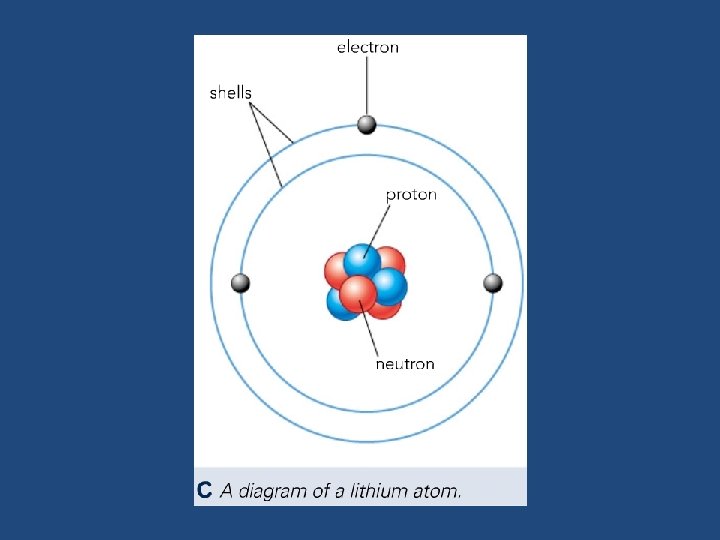
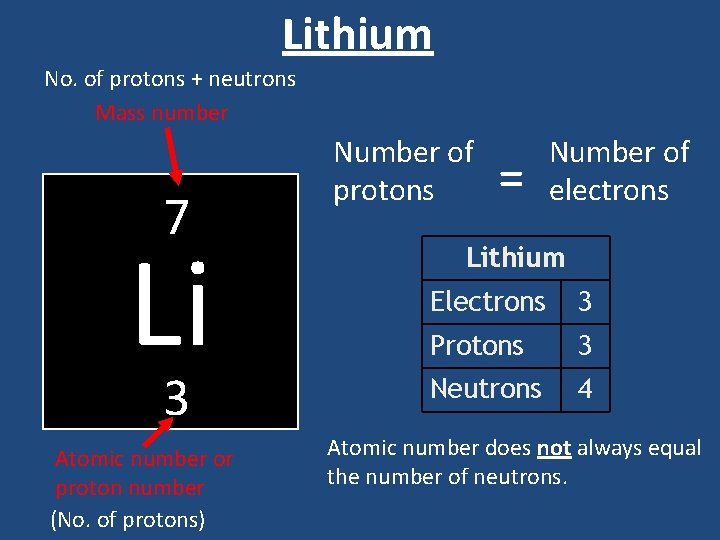
Lithium No. of protons + neutrons Mass number 7 Li 3 Atomic number or proton number (No. of protons) Number of protons = Number of electrons Lithium Electrons 3 Protons 3 Neutrons 4 Atomic number does not always equal the number of neutrons.

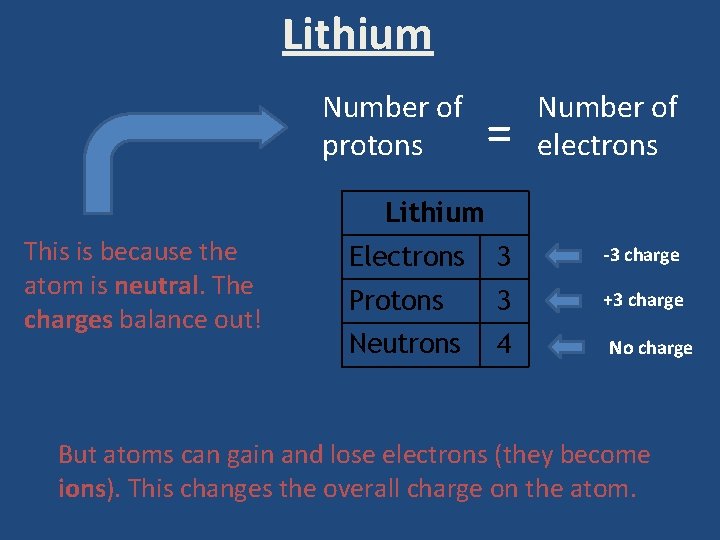
Lithium Number of protons = Number of electrons Electrons 3 -3 charge Protons 3 +3 charge Neutrons 4 No charge Lithium This is because the atom is neutral. The charges balance out! But atoms can gain and lose electrons (they become ions). This changes the overall charge on the atom.

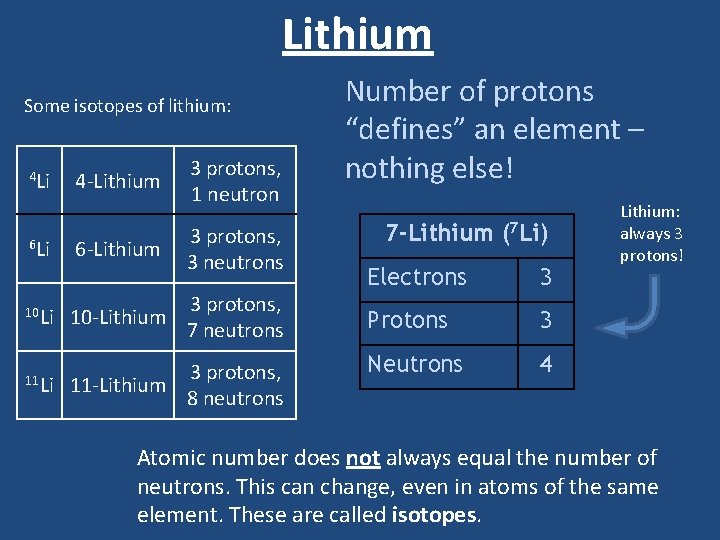
Lithium Some isotopes of lithium: 4 Li 6 Li 4 -Lithium 6 -Lithium 3 protons, 1 neutron 3 protons, 3 neutrons 3 protons, 7 neutrons 10 Li 10 -Lithium 11 Li 3 protons, 11 -Lithium 8 neutrons Number of protons “defines” an element – nothing else! 7 -Lithium (7 Li) Electrons 3 Protons 3 Neutrons 4 Lithium: always 3 protons! Atomic number does not always equal the number of neutrons. This can change, even in atoms of the same element. These are called isotopes.

How many electrons in? 1. Hydrogen 2. Helium 3. Beryllium 4. Carbon 5. Oxygen Use your periodic table!