Atom Definition the smallest particle of any element





















- Slides: 33

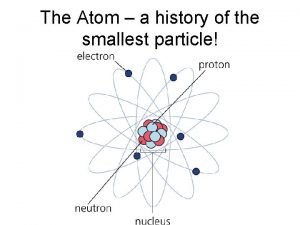
Atom Definition: the smallest particle of any element that retains the properties of that element.

Practice quiz question: What is the smallest particle of magnesium that retains the properties of magnesium?

Interesting side note… Atoms are fantastically durable. Because they last so long, atoms really get around! Every atom you possess has almost certainly passed through several stars and been part of millions of organisms on its way to becoming you.

Interesting side note… It has been suggested that up to a billion of each person’s atoms once belonged to Shakespeare. Another billion came from Beethoven. You probably have some atoms from any historical figure you can think of!

Nowadays, with the use of tunneling electron microscopes, we can sort of “see” atoms… image of copper atoms

This is an image of silicon atoms arranged on a face of a crystal. It is impossible to "see" atoms this way using ordinary light. The image was made by a Scanning Tunneling Microscope, a device that "feels" the cloud of electrons that form the outer surface of atoms, like a phonograph needle feels the grooves in a record.

These electron microscopes were invented in the mid-1900’s. How did scientists figure out atomic structure without being able to look at any atoms?

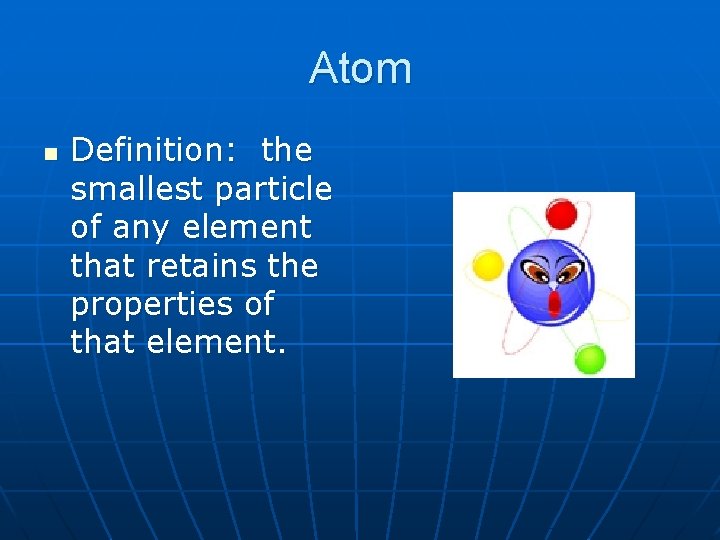
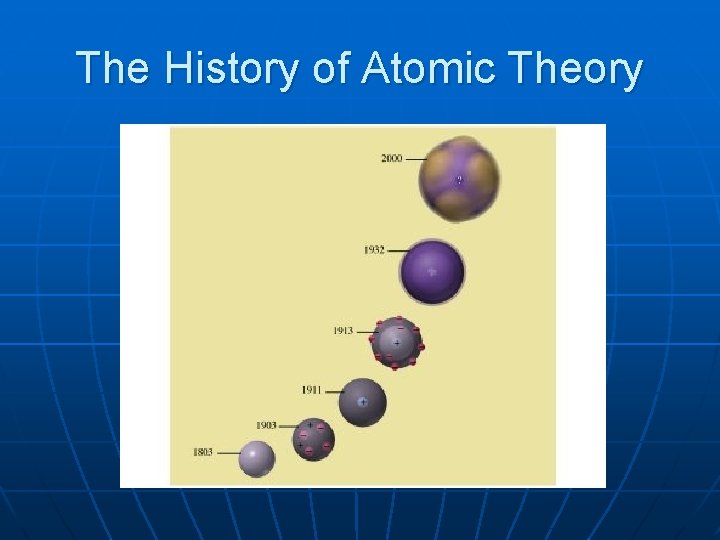
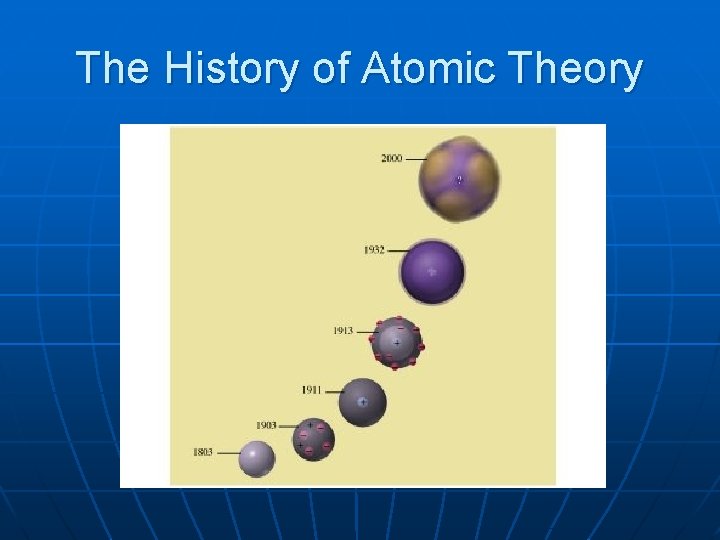
The History of Atomic Theory

But first… What is a theory in science? A theory is a well-tested explanation of what happens in nature. In layman’s terms, if something is said to be “just a theory, ” it usually means that it is a mere guess, or is unproven. But in scientific terms, a theory implies that something has been welltested and verified many times by multiple groups of scientists.

And while we’re at it… A scientific law is a statement of something that seems to be true in the natural world. Example: The Law of Gravity. Newton could use this law to predict the behavior of a dropped object, but he couldn't explain why it happened.

Einstein’s Theory of Relativity explains gravity as a curve in the fabric of space-time:

Remember! A law states or describes what happens in nature. A theory explains what happens. Both are well-tested by experiments. Memory hint: “explanation starts with an “e” and “theory” has an “e”.

True or False? The difference between a scientific law and a scientific theory is that a law has been proven, but a theory has not been proven.

The History of Atomic Theory

Democritus ancient Greek philosopher, not scientist first to suggest that matter is made of tiny particles called “atomos” = Greek for “indivisible” ideas were rejected by Aristotle, who was very influential, and therefore forgotten for two thousand years.

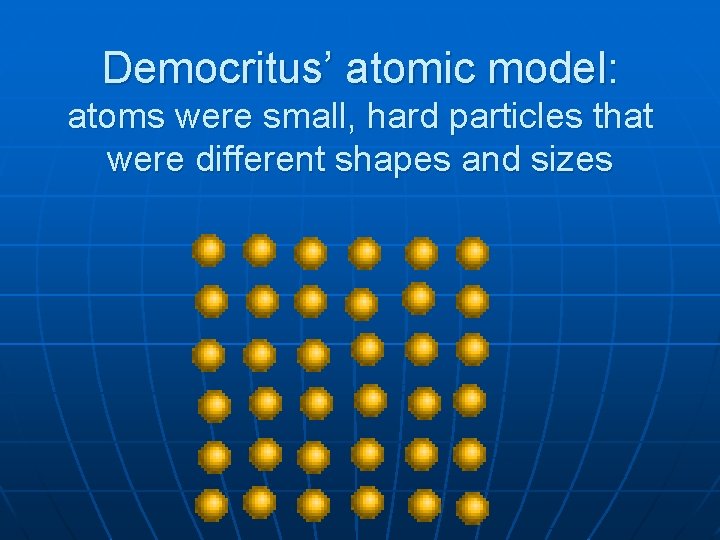
Democritus’ atomic model: atoms were small, hard particles that were different shapes and sizes

John Dalton: First to propose an atomic theory based on science: 1. Each element is composed of small particles called atoms. 2. All atoms of a given element are identical*; the atoms of different elements are different. 3. Atoms are not created or destroyed in chemical reactions. 4. Compounds are formed when atoms of more than one element combine.

John Dalton: First to propose an atomic theory based on science: 1. Each element is composed of small particles called atoms. 2. All atoms of a given element are identical*; the atoms of different elements are different. 3. Atoms are not created or destroyed in chemical reactions. 4. Compounds are formed when atoms of more than one element combine. * We now know this part is not true!

Dalton’s atomic model: a tiny sphere that is indivisible

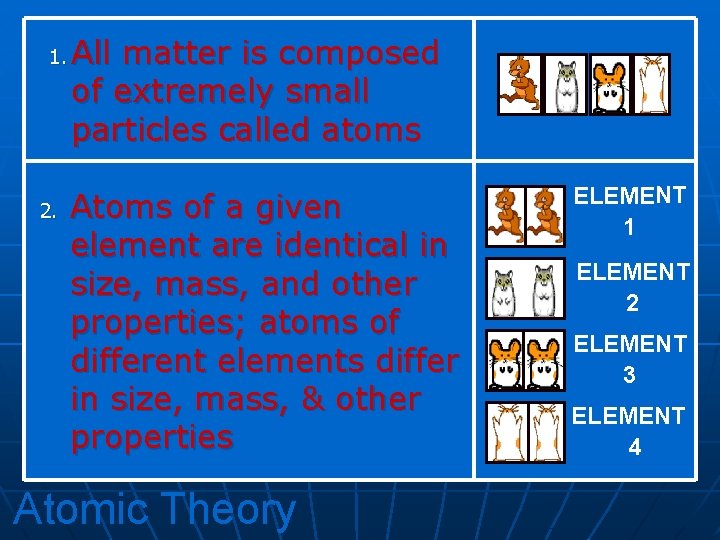
1. 2. All matter is composed of extremely small particles called atoms Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, & other properties Atomic Theory ELEMENT 1 ELEMENT 2 ELEMENT 3 ELEMENT 4

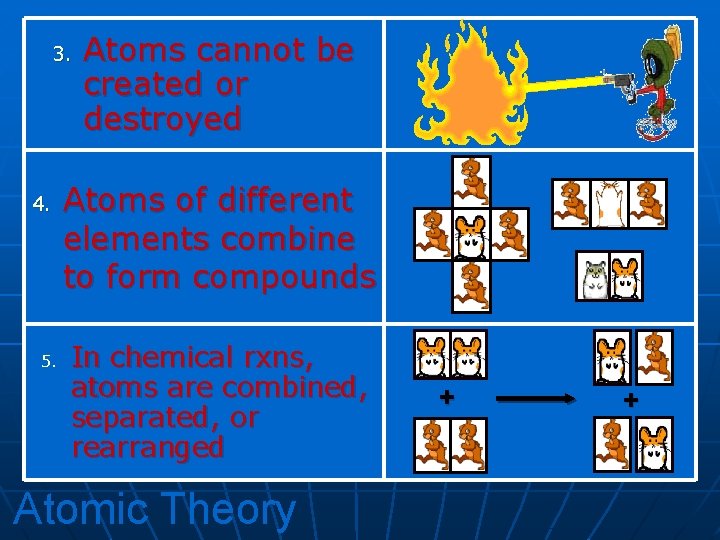
3. 4. 5. Atoms cannot be created or destroyed Atoms of different elements combine to form compounds In chemical rxns, atoms are combined, separated, or rearranged Atomic Theory + +

J. J. Thomson: First to suggest that there were particles smaller than the atom. Discovered the electron. Developed the “plum pudding model. ”

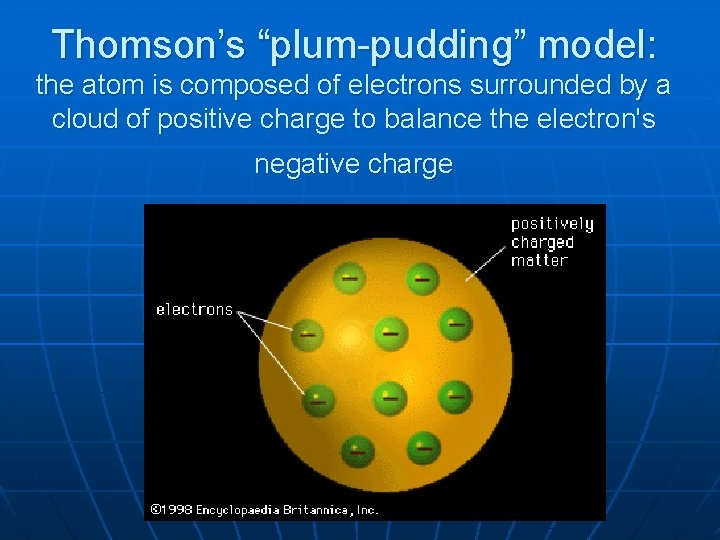
Thomson’s “plum-pudding” model: the atom is composed of electrons surrounded by a cloud of positive charge to balance the electron's negative charge

Ernest Rutherford gold foil experiment discovered the nucleus, and realized that it was very dense and positively charged suggested that most of the atom is empty space

Gold Foil Experiment: Rutherford shot tiny positively-charged alpha particles through a thin sheet of gold foil What Rutherford expected: What really happened:

Gold Foil Experiment: Some of the alpha particles bounced back. “It was as if you fired a 15 -inch cannon ball at a piece of tissue paper and it came back and hit you. ” What Rutherford expected: What really happened:

Such huge deflections could mean only one thing: some of the alpha particles had run into massive concentrations of positive charge and, since like charges repel, had been hurled straight back by them.

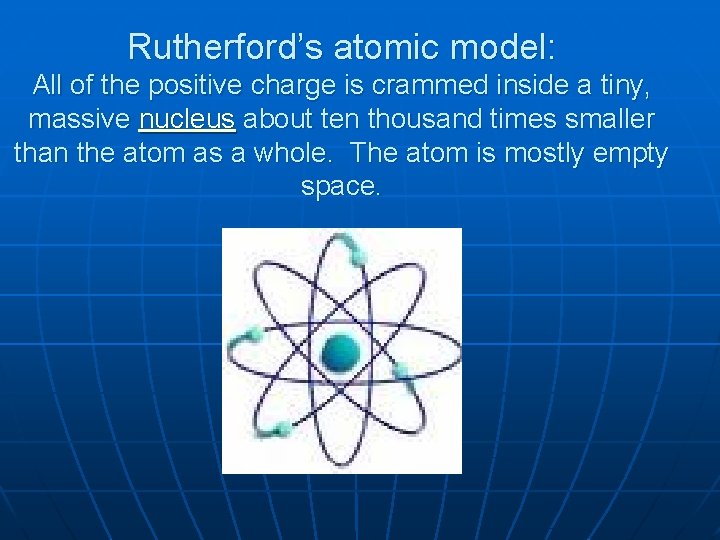
Rutherford’s atomic model: All of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole. The atom is mostly empty space.

Animation of Rutherford’s experiment http: //www. mhhe. com/physsci/chem istry/essentialchemistry/flash/ruther 14. swf

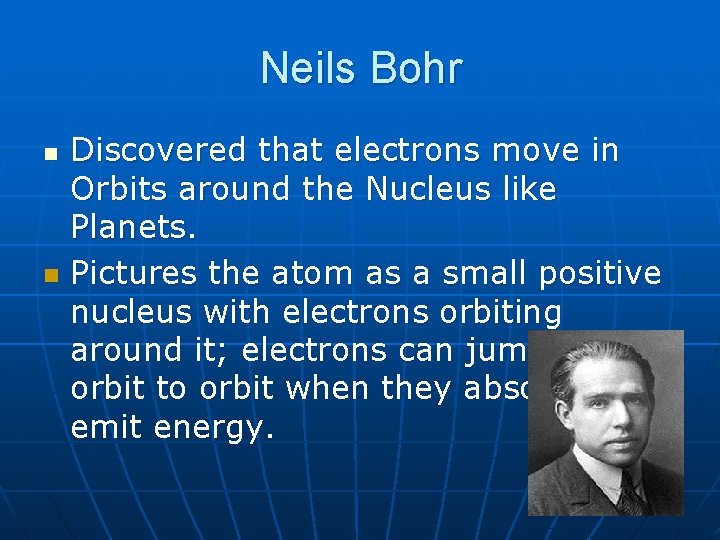
Neils Bohr Discovered that electrons move in Orbits around the Nucleus like Planets. Pictures the atom as a small positive nucleus with electrons orbiting around it; electrons can jump from orbit to orbit when they absorb or emit energy.

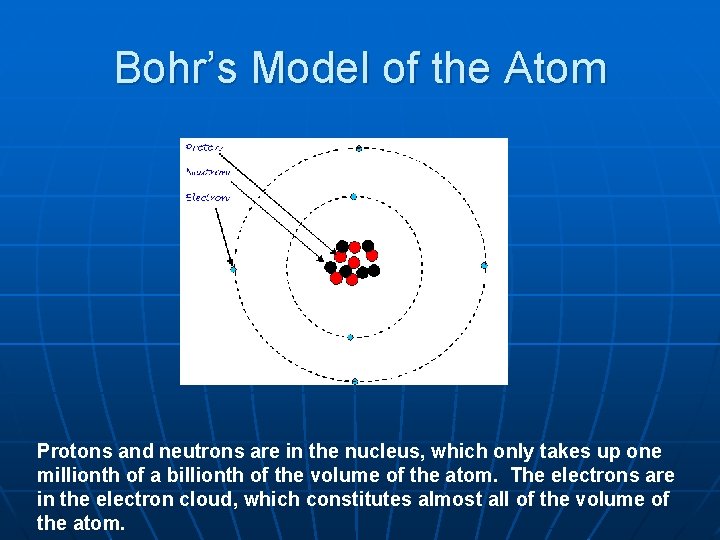
Bohr’s Model of the Atom Protons and neutrons are in the nucleus, which only takes up one millionth of a billionth of the volume of the atom. The electrons are in the electron cloud, which constitutes almost all of the volume of the atom.

In fact, if an atom were expanded to the size of a cathedral, the nucleus would be about the size of a housefly!

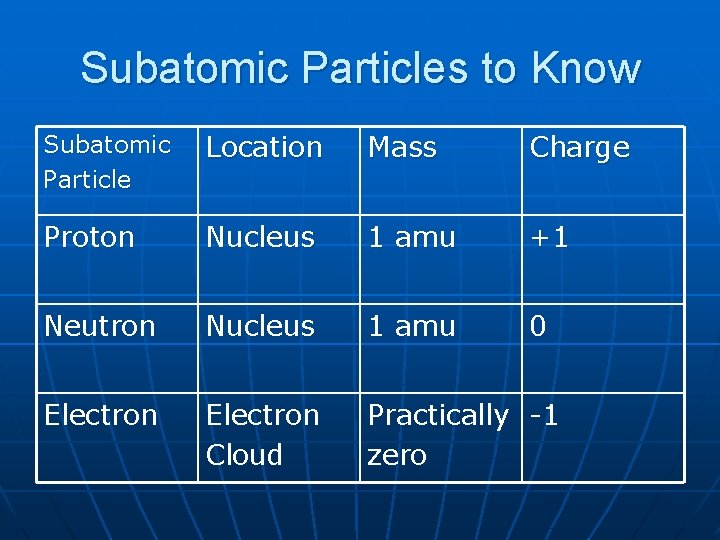
Subatomic Particles to Know Subatomic Particle Location Mass Charge Proton Nucleus 1 amu +1 Neutron Nucleus 1 amu 0 Electron Cloud Practically -1 zero
 Smallest particle of an element
Smallest particle of an element Smallest particle on earth
Smallest particle on earth Atoms are small hard particles
Atoms are small hard particles Whats the smallest particle of an element
Whats the smallest particle of an element Smallest particle
Smallest particle Is an atom the smallest unit of matter
Is an atom the smallest unit of matter What would happen
What would happen Particle atom molecule
Particle atom molecule Atom
Atom Language refers to the
Language refers to the The smallest unit of a textile material is called a:
The smallest unit of a textile material is called a: Solid element particle diagram
Solid element particle diagram Representative particle
Representative particle Representative particle examples
Representative particle examples Smallest non metal
Smallest non metal The smallest piece of an element
The smallest piece of an element What is the smallest part of an element
What is the smallest part of an element The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Teori perkembangan atom dalton
Teori perkembangan atom dalton There are some cake
There are some cake Any to any connectivity
Any to any connectivity Informational probes adalah
Informational probes adalah Expanded octet
Expanded octet Pure element -only one type of atom present
Pure element -only one type of atom present Identify a contextual selector that matches any element.
Identify a contextual selector that matches any element. Difference between signal element and data element
Difference between signal element and data element Signal element vs data element
Signal element vs data element Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Chúa sống lại
Chúa sống lại