What is an Atom Atom Smallest unit of


- Slides: 16


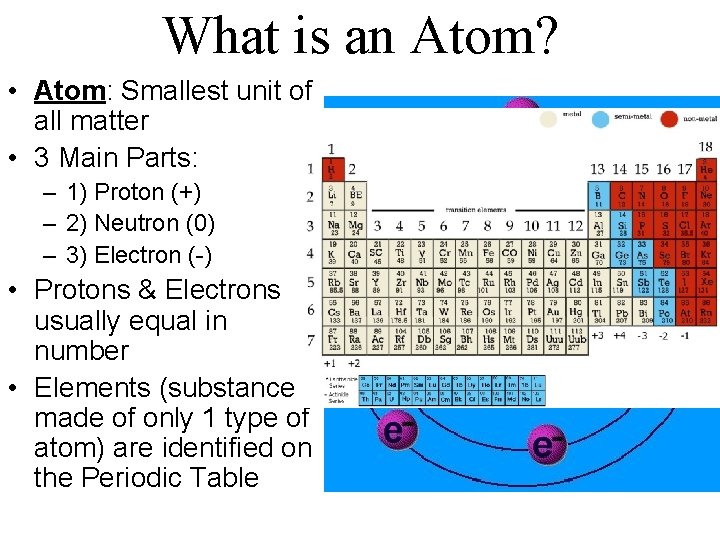
What is an Atom? • Atom: Smallest unit of all matter • 3 Main Parts: – 1) Proton (+) – 2) Neutron (0) – 3) Electron (-) • Protons & Electrons usually equal in number • Elements (substance made of only 1 type of atom) are identified on the Periodic Table

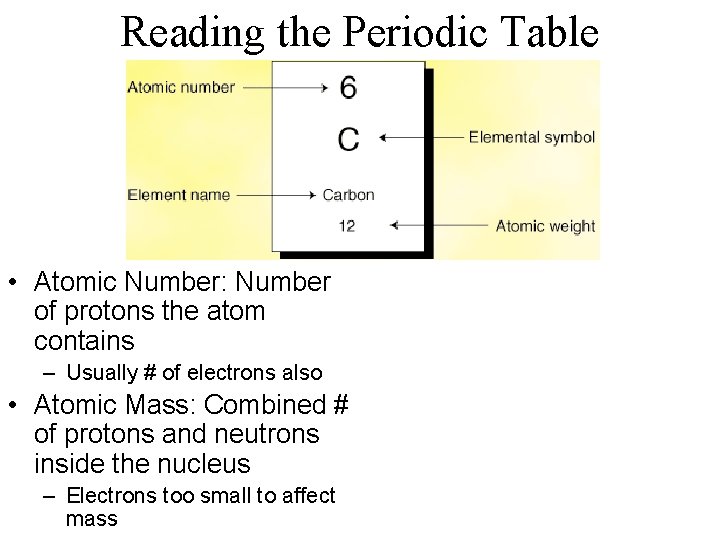
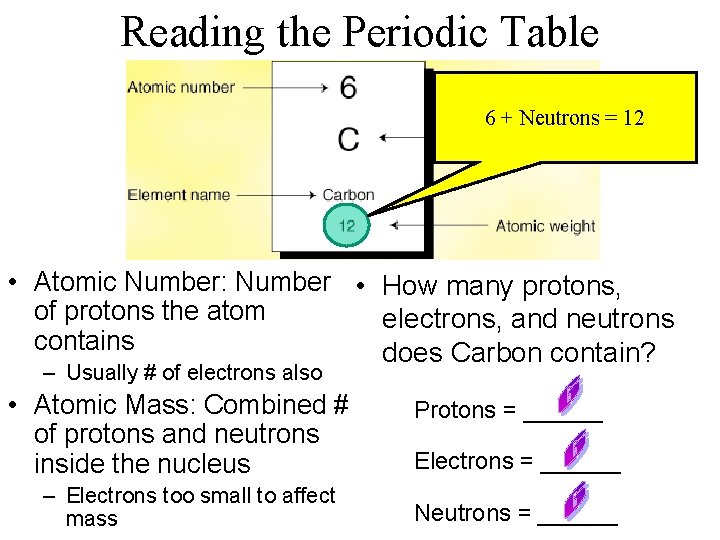
Reading the Periodic Table • Atomic Number: Number of protons the atom contains – Usually # of electrons also • Atomic Mass: Combined # of protons and neutrons inside the nucleus – Electrons too small to affect mass

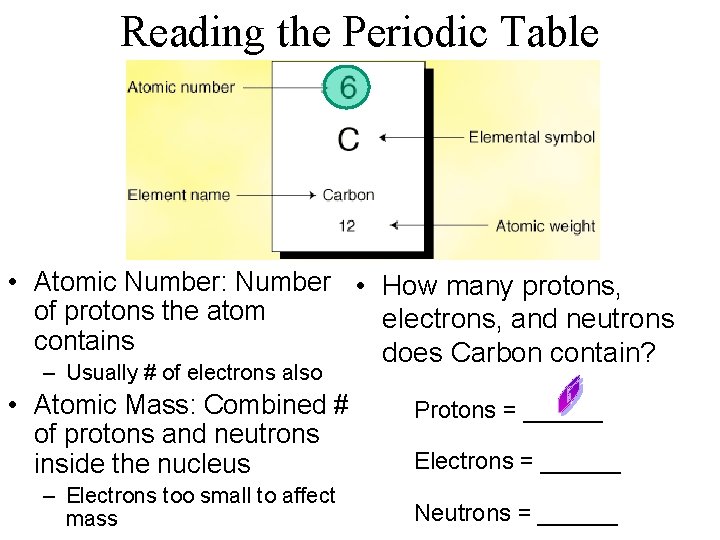
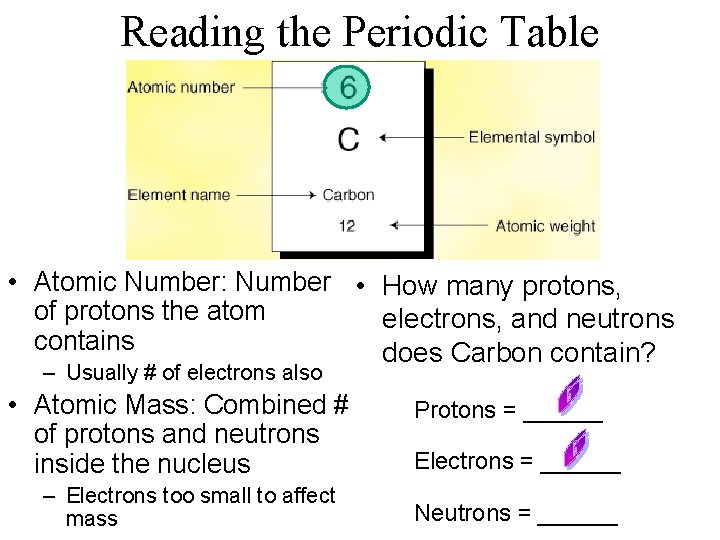
Reading the Periodic Table • Atomic Number: Number • How many protons, of protons the atom electrons, and neutrons contains does Carbon contain? – Usually # of electrons also • Atomic Mass: Combined # of protons and neutrons inside the nucleus – Electrons too small to affect mass Protons = ______ Electrons = ______ Neutrons = ______

Reading the Periodic Table • Atomic Number: Number • How many protons, of protons the atom electrons, and neutrons contains does Carbon contain? – Usually # of electrons also • Atomic Mass: Combined # of protons and neutrons inside the nucleus – Electrons too small to affect mass Protons = ______ Electrons = ______ Neutrons = ______

Reading the Periodic Table Protons 6 + Neutrons = 12 • Atomic Number: Number • How many protons, of protons the atom electrons, and neutrons contains does Carbon contain? – Usually # of electrons also • Atomic Mass: Combined # of protons and neutrons inside the nucleus – Electrons too small to affect mass Protons = ______ Electrons = ______ Neutrons = ______

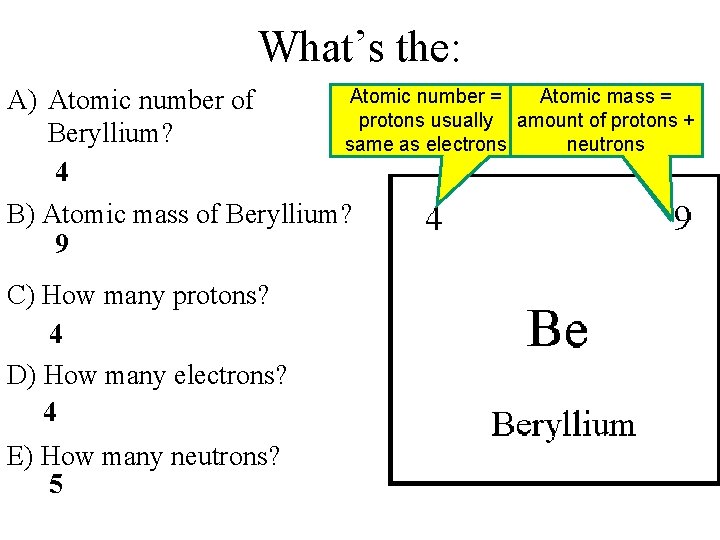
What’s the: Atomic number = A) Atomic number of Atomic number = protons usually amount of protons Beryllium? same as electrons 4 B) Atomic mass of Beryllium? 9 C) How many protons? 4 D) How many electrons? 4 E) How many neutrons? 5 Atomic mass = amount of protons + neutrons

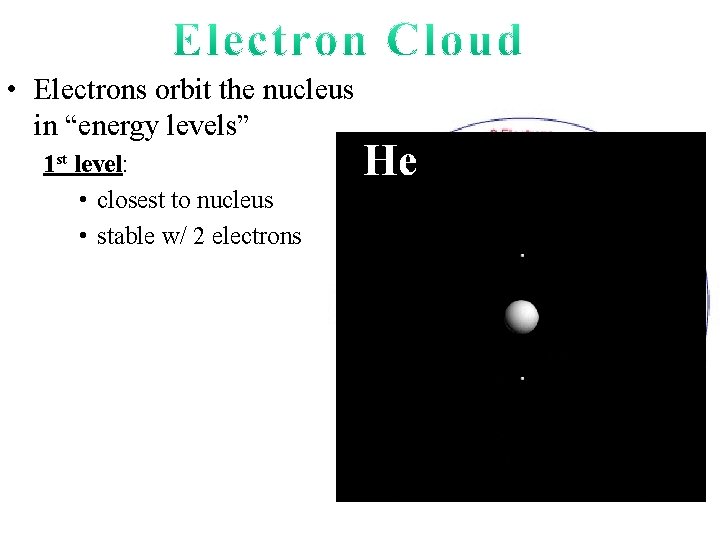
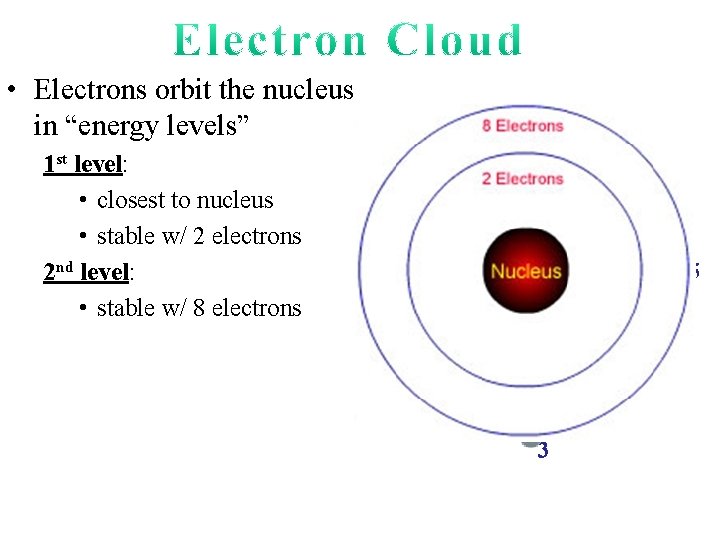
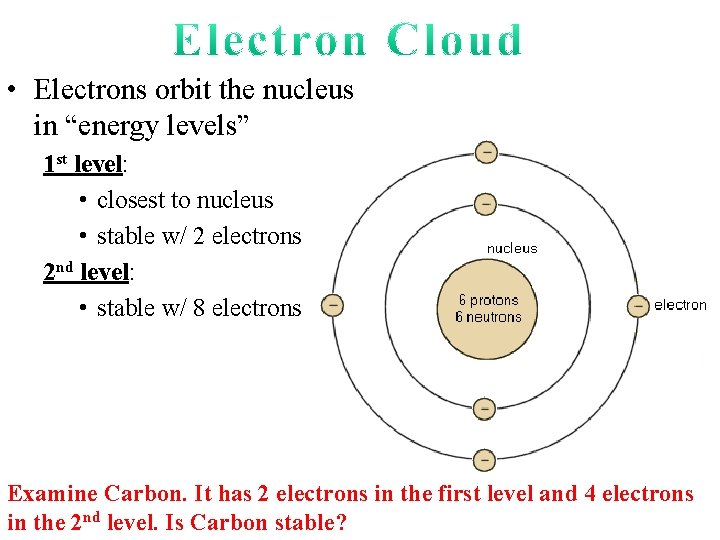
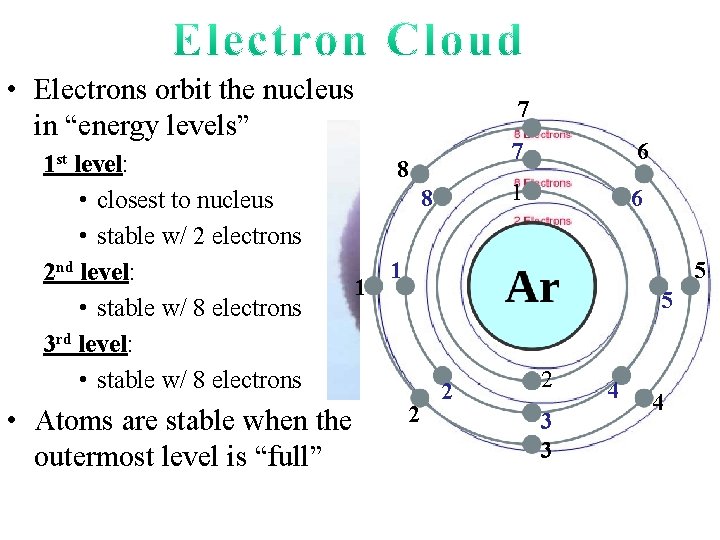
• Electrons orbit the nucleus in “energy levels” 1 st level: • closest to nucleus • stable w/ 2 electrons

• Electrons orbit the nucleus in “energy levels” 1 st level: • closest to nucleus • stable w/ 2 electrons 2 nd level: • stable w/ 8 electrons 7 1 8 6 5 1 2 4 2 3

• Electrons orbit the nucleus in “energy levels” 1 st level: • closest to nucleus • stable w/ 2 electrons 2 nd level: • stable w/ 8 electrons Examine Carbon. It has 2 electrons in the first level and 4 electrons in the 2 nd level. Is Carbon stable?

• Electrons orbit the nucleus in “energy levels” 1 st level: • closest to nucleus • stable w/ 2 electrons 2 nd level: • stable w/ 8 electrons 3 rd level: • stable w/ 8 electrons • Atoms are stable when the outermost level is “full” 7 8 8 1 7 6 1 6 5 1 5 2 2 2 3 3 4 4

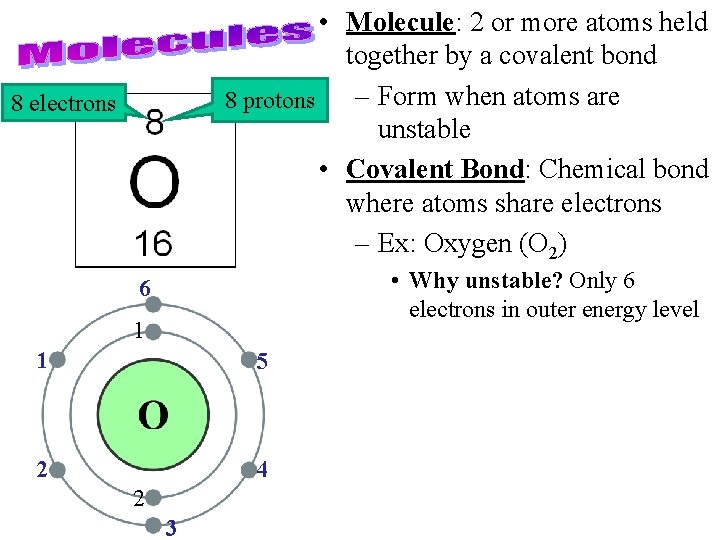
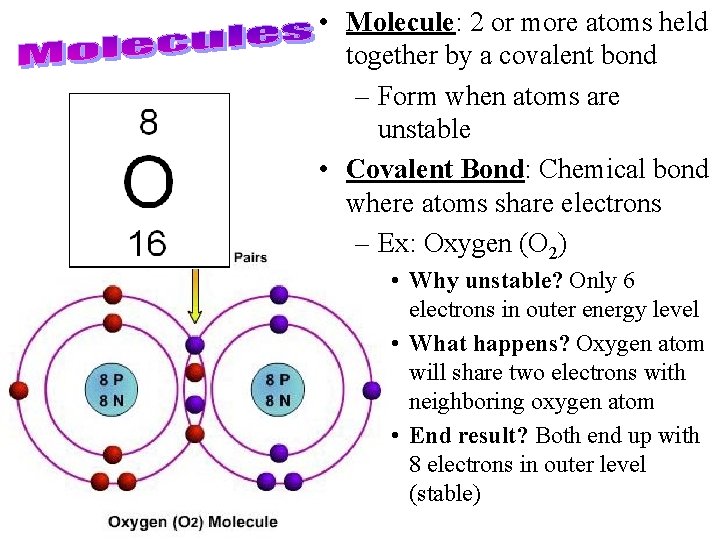
• Molecule: 2 or more atoms held together by a covalent bond – Form when atoms are 8 protons unstable • Covalent Bond: Chemical bond where atoms share electrons – Ex: Oxygen (O 2) 8 electrons • Why unstable? Only 6 electrons in outer energy level 6 1 1 5 2 4 2 3

• Molecule: 2 or more atoms held together by a covalent bond – Form when atoms are unstable • Covalent Bond: Chemical bond where atoms share electrons – Ex: Oxygen (O 2) • Why unstable? Only 6 electrons in outer energy level • What happens? Oxygen atom will share two electrons with neighboring oxygen atom • End result? Both end up with 8 electrons in outer level (stable)

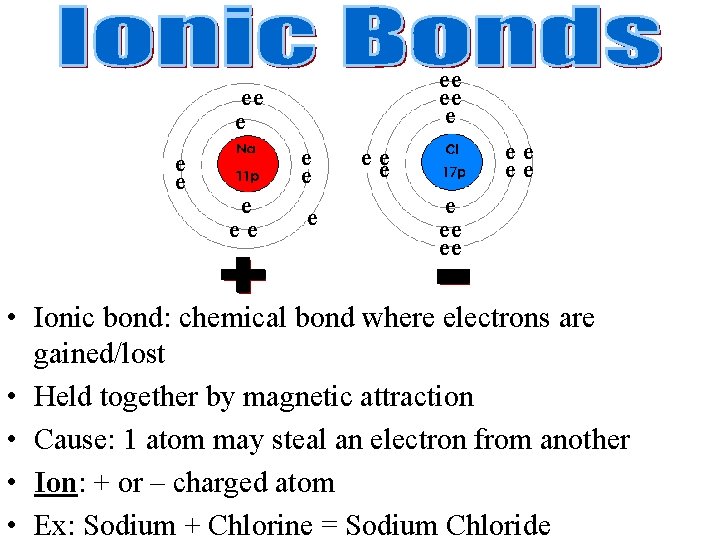
ee ee e ee e ee ee • Ionic bond: chemical bond where electrons are gained/lost • Held together by magnetic attraction • Cause: 1 atom may steal an electron from another • Ion: + or – charged atom • Ex: Sodium + Chlorine = Sodium Chloride

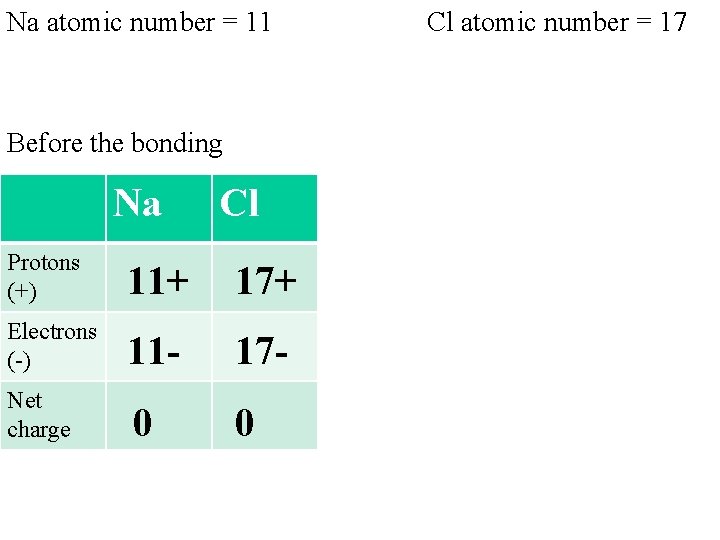
Na atomic number = 11 Cl atomic number = 17 Before the bonding After the bond Na Protons (+) Electrons (-) Net charge 11+ 110 Cl Na Cl 17+ Protons (+) 11+ 17 - Electrons (-) 10 - 18 - 0 Net charge +1 -1

Recap 1) Name the 3 subatomic particles. 2) Which subatomic particles are found inside the nucleus? 3) The atomic number usually allows us to determine the amount of which two subatomic particles? 4) If an atom has the atomic mass of 14 and the atomic number of 6, how many protons, neutrons, and electrons does the atom contain? 5) After an ionic bond is formed, what is the charge of the atom that gained an electron? 6) Which type of chemical bond shares electrons?