Structure of Atom Atom Smallest particle of an









- Slides: 17

Structure of Atom

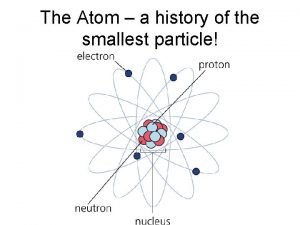
Atom Smallest particle of an element Atomic Structure – arrangement of smaller particles within an atom

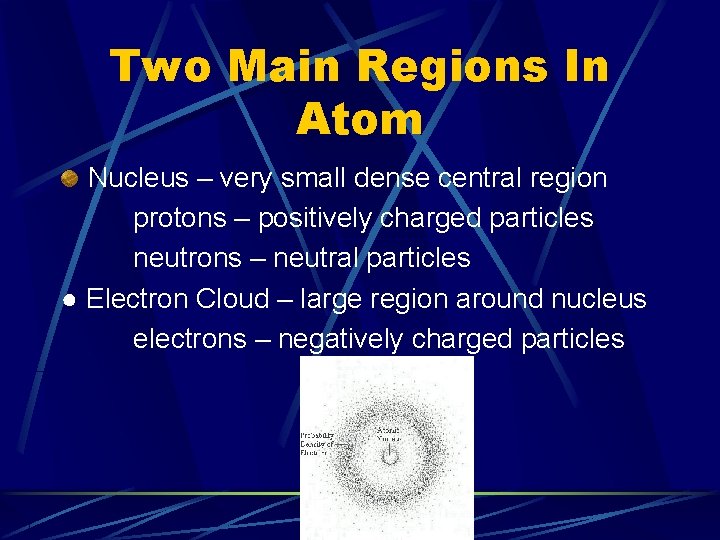
Two Main Regions In Atom Nucleus – very small dense central region protons – positively charged particles neutrons – neutral particles ● Electron Cloud – large region around nucleus electrons – negatively charged particles

Discovery of Electrons JJ Thomson – used Crooke’s Tube (cathode ray tube) Gas under low pressure in a sealed glass tube Has a positive end (Anode) and negative end (Cathode) When anode and cathode are connected to the + and – terminals of a battery, the tube produces a beam of particles called electrons



Thomson’s Atom Plum-pudding model Since total atom is neutral, there must be an equal number of + and - charges Atom is a positively charged area with negative electrons randomly spread throughout

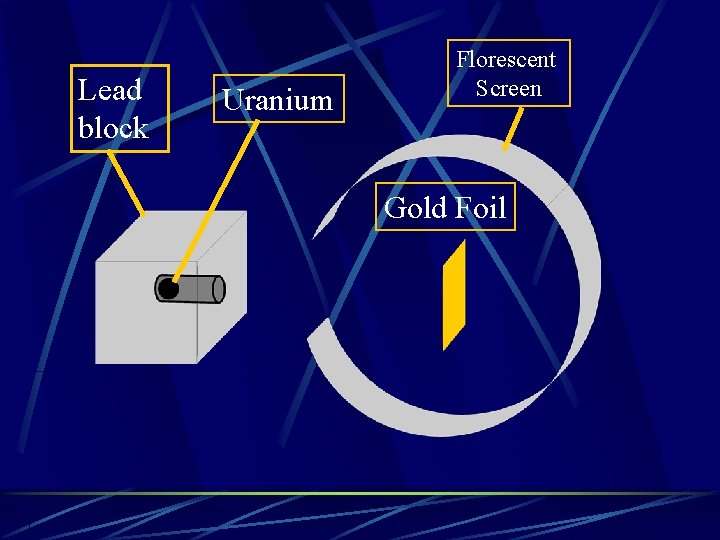
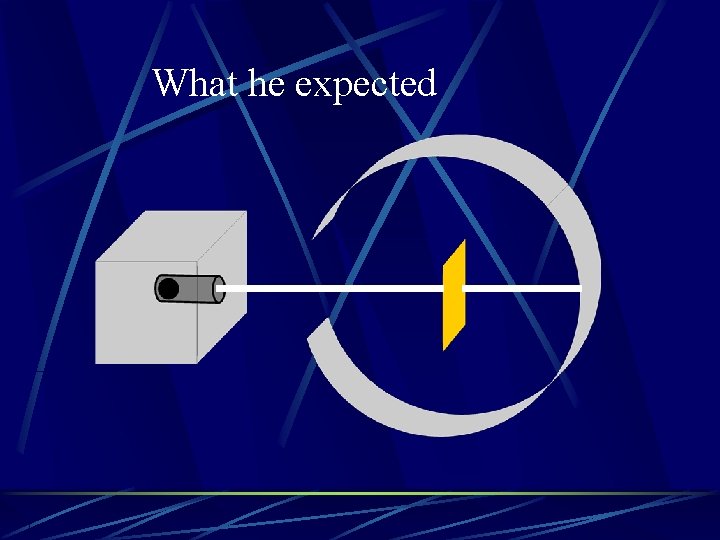
Nucleus Ernest Rutherford – Gold foil experiment Shot positive charged particles at a very thin piece of gold foil

Lead block Uranium Florescent Screen Gold Foil

What he expected

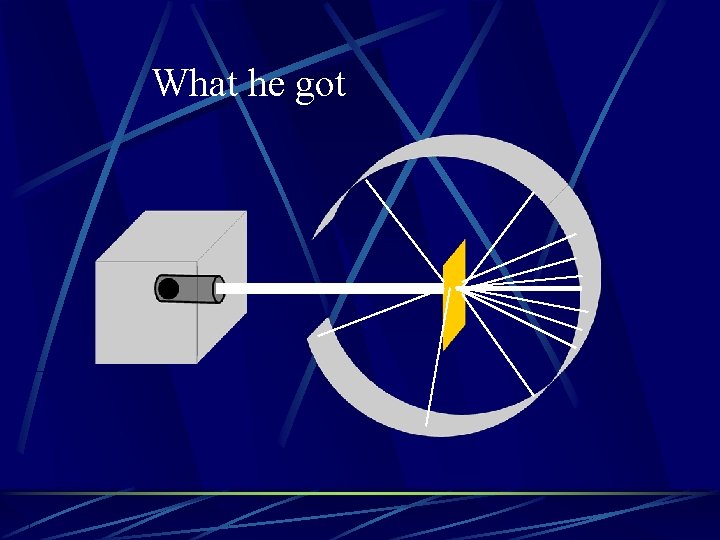
What he got


How he explained it Atom is mostly empty space Small dense, positive piece at center Alpha particles are deflected by it if they get close enough +

Rutherford’s Model Of Atom Positively charged nucleus containing protons and neutrons with negatively charged particles in orbit around it Electrons stay in orbit due to attraction between + and – charges

Problems With Rutherford Model Charged particle when moving in a curved path give off light so they lose energy If they lose energy, they would gradually get closer and closer to the nucleus and crash into it

Bohr Model of Atom Similar to Rutherford model, but in this model electrons orbit in specific, well defined energy level A gain or loss of energy moves the electron from one energy level to another Positively charged protons and neutral neutrons held together tightly by the Strong Nuclear Force

 Smallest particle
Smallest particle Whats the smallest particle of matter
Whats the smallest particle of matter What is the smallest part of an element
What is the smallest part of an element Whats the smallest particle of an element
Whats the smallest particle of an element Atoms are small,hard particles
Atoms are small,hard particles Whats the smallest particle of matter
Whats the smallest particle of matter The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Particle atom molecule
Particle atom molecule What is the smallest unit of a metallic bond
What is the smallest unit of a metallic bond What's the smallest piece of an element
What's the smallest piece of an element Kelemahan atom dalton
Kelemahan atom dalton Describe the structure of an atom?
Describe the structure of an atom? What is fine structure of hydrogen atom
What is fine structure of hydrogen atom Section 1 structure of the atom
Section 1 structure of the atom Section 1 structure of the atom
Section 1 structure of the atom Structure of periodic table
Structure of periodic table Atomic packing factor of fcc
Atomic packing factor of fcc Atom and its structure
Atom and its structure