The Atom a history of the smallest particle














- Slides: 23

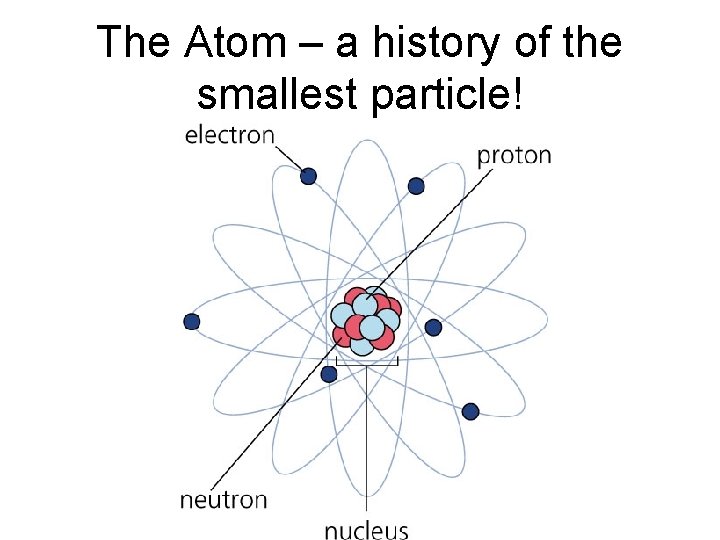
The Atom – a history of the smallest particle!

• Ancient Greeks were the first to imagine the existence of tiny particles that could not be divided or broken into anything smaller • The Greek word for indivisible is “Atomos” this is where we get the word “Atom” • However the Greeks did not have the means to prove the existence of atoms through experiment

John Dalton • The 12 year old headmaster! • In 1808 this English Chemist released a book on atomic theory to explain experiments he had done with gases • He was widely praised and honoured but was very shy and shunned glory

Summary of Dalton’s atomic theory 1. All matter is made up of very small particles called atoms 2. All atoms are indivisible they cannot be broken into smaller particles 3. Atoms cannot be created or destroyed • • Spot the incorrect point!? Of course we know atoms can be broken down into electrons, neutrons and protons we will see now how we found this out!

Discovery of the electron • William Crookes in 1875 • Proved that electrons existed by showing how cathode rays behaved in a vacuum tube

• A battery is connected at its negative end called the CATHODE to a vacuum tube • When the current is turned on a glow comes from the tube • Crookes showed that this was caused by some kind of radiation by placing a maltese cross in the path of the rays and showing that a shadow was formed


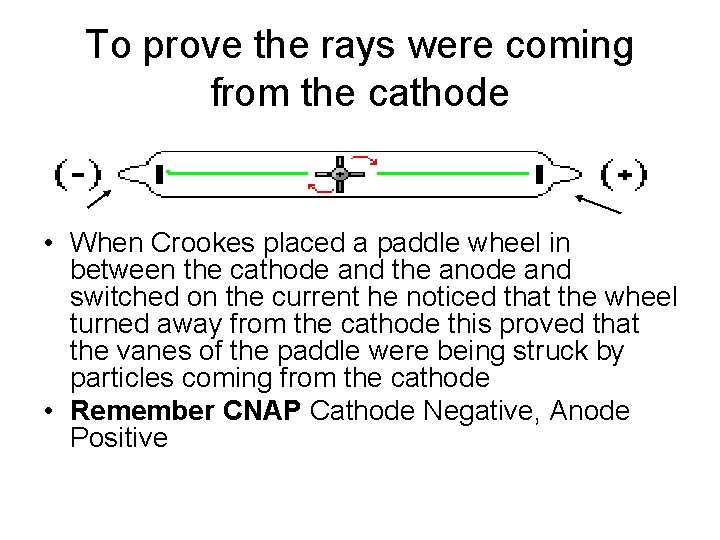
To prove the rays were coming from the cathode Cathode Anode • When Crookes placed a paddle wheel in between the cathode and the anode and switched on the current he noticed that the wheel turned away from the cathode this proved that the vanes of the paddle were being struck by particles coming from the cathode • Remember CNAP Cathode Negative, Anode Positive

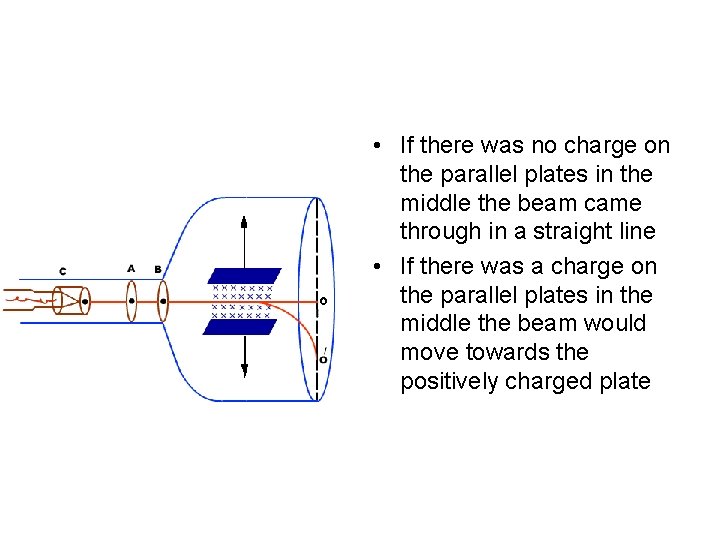
J. J. Thomson • Englishman in 1897 who showed that the cathode rays were made of small charged particles

• If there was no charge on the parallel plates in the middle the beam came through in a straight line • If there was a charge on the parallel plates in the middle the beam would move towards the positively charged plate

Thomson’s conclusions • Thomson concluded that since the cathode rays were attracted to the positive plate they must be made of negatively charged particles as opposites attract • These negatively charged particles were called ELECTRONS • CATHODE RAYS are streams of negatively charged electrons

The Plum Pudding Model • A very simple model of an atom proposed by Thomson • Since atoms are neutral and he knew electrons were negative he correctly said that there must also be positive charges • He proposed that electrons were dotted in a positive cloud like raisins in a plum pudding!

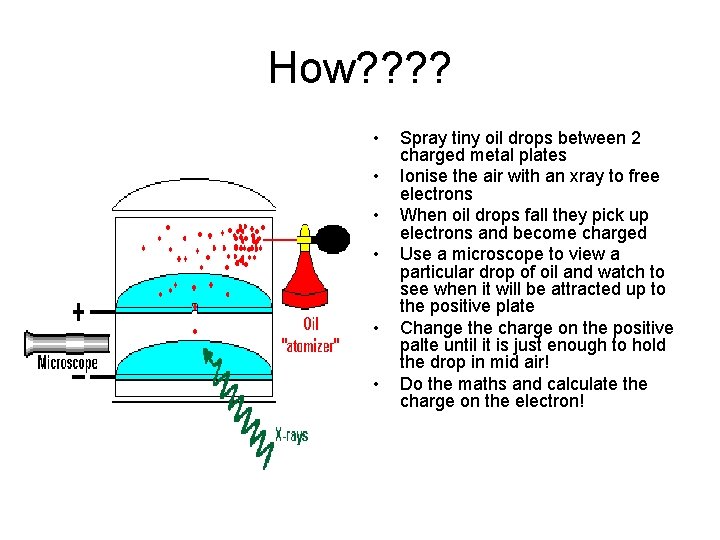
The tiniest of particles! • In 1909 an American named Robert Millikan performed the OIL DROP EXPERIMENT to find the size of the charge on an electron and he then found the mass of an electron • He found an electron has a mass of 9. 1 x 10 -39 kg! very small

How? ? • • • Spray tiny oil drops between 2 charged metal plates Ionise the air with an xray to free electrons When oil drops fall they pick up electrons and become charged Use a microscope to view a particular drop of oil and watch to see when it will be attracted up to the positive plate Change the charge on the positive palte until it is just enough to hold the drop in mid air! Do the maths and calculate the charge on the electron!

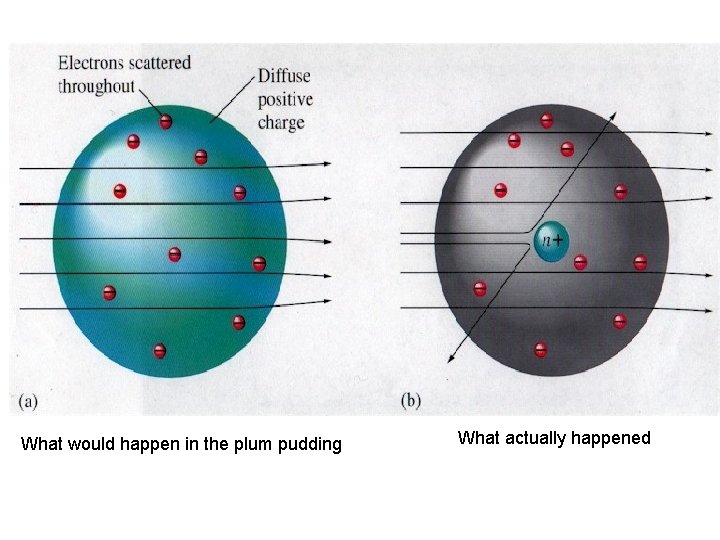
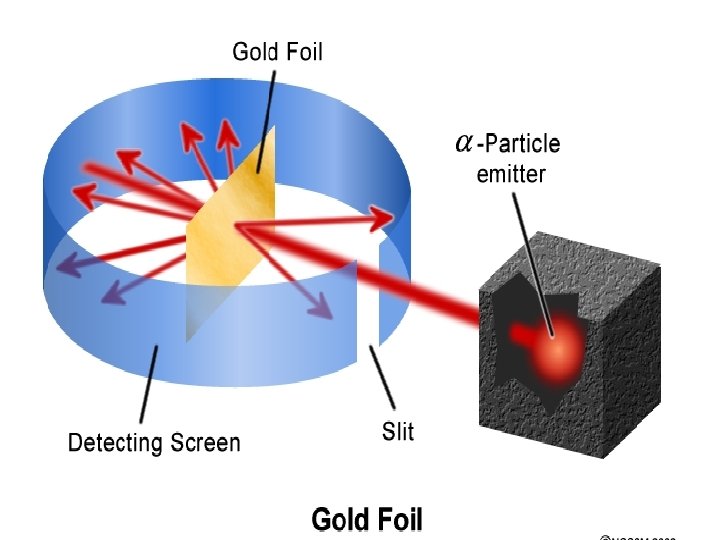
Discovery of the nucleus • Ernest Rutherford from New Zealand 1909 • Used a radioactive source to fire alpha particles (positively charged particles) at a sheet of gold foil • Expected that the particles would be slightly deflected as they passed through the “plum pudding” atoms in the gold foil

• ! What would happen in the plum pudding What actually happened


• Most alpha particles went straight through the gold foil, some were slightly deflected but others bounced right back and travelled back along there own path like they had bounced off something • The only way to explain this was that the positive part of the atoms in the gold foil was concentrated in one small dense core – the NUCLEUS was discovered!

Discovery of the Proton • Rutherford and his team found that when small atoms like oxygen and nitrogen were bombarded they gave off positive particles but heavier atoms such as gold didn’t • Reason was that bombarding broke up the nucleus in small atoms and released protons but in bigger atoms there were too many protons and the strong positive charge repelled the alpha particles

Discovery of the Neutron • James Chadwick • If the nucleus was all protons it would fall apart as they would repel each other • Chadwick a former student of Rutherfords bombarded beryllium with alpha particles and found that neutral particles were being knock out of the beryllium

Importance of neutrons • Stop nuclei coming apart • Are used to split atoms of uranium to release nuclear energy in the atomic bomb and nuclear reactors

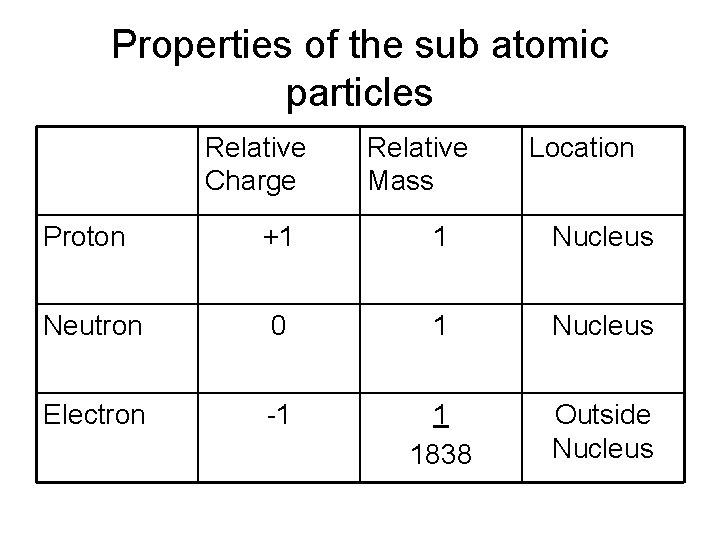
Properties of the sub atomic particles Relative Charge Relative Mass Location Proton +1 1 Nucleus Neutron 0 1 Nucleus Electron -1 1 1838 Outside Nucleus

2007 Question 11 (a) In 1910 Rutherford (pictured right) and his coworkers carried out an experiment in which thin sheets of gold foil were bombarded with alpha particles. The observations made during the experiment led to the discovery of the atomic nucleus. (i) Describe the model of atomic structure which existed immediately prior to this experiment. (7) (ii) In this experiment it was observed that most of the alpha particles went straight through the gold foil. Two other observations were made. State these other observations and explain how each helped Rutherford deduce that the atom has a nucleus. (12)
 Smallest particle
Smallest particle Smallest particle of an element
Smallest particle of an element The smallest particle of an element is called
The smallest particle of an element is called Smallest particle
Smallest particle Atoms are small hard particles
Atoms are small hard particles Whats the smallest particle of matter
Whats the smallest particle of matter Particle atom molecule
Particle atom molecule The smallest unit of matter
The smallest unit of matter Atom
Atom The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Democritus atom modeli
Democritus atom modeli History of the atom webquest
History of the atom webquest History of atomic models
History of atomic models History of the atom scientists
History of the atom scientists History of the atom john dalton
History of the atom john dalton Teori atom democritus
Teori atom democritus John dalton billiard ball model
John dalton billiard ball model Dalton thomson rutherford bohr timeline
Dalton thomson rutherford bohr timeline Also history physical
Also history physical Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào