Q Whats an atom l The smallest piece









- Slides: 20

Q: What’s an atom? l The smallest piece of matter that still has all the properties and characteristics of that type of matter l EX. An atom of gold still has the same chemical and physical properties as a chunk of gold

Q: What’s an atom? l Brownian Motion ¡ Perpetual movement of particles (atoms jiggling/vibrating) ¡ Named after Robert Brown (Scottish botanist).

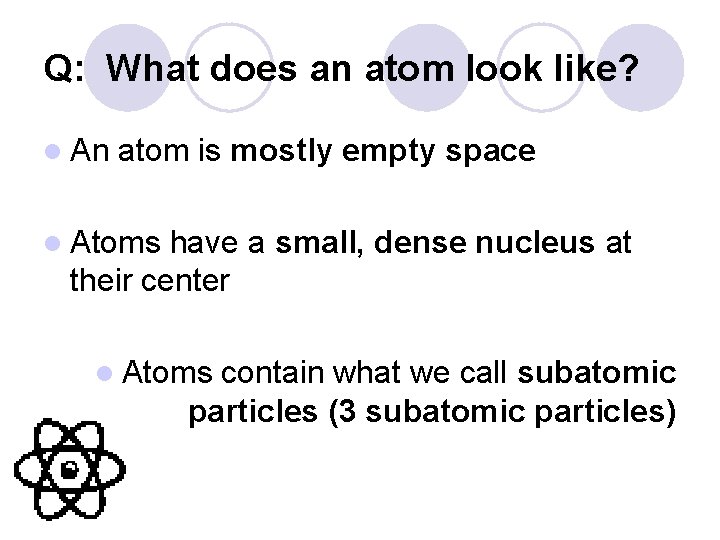
Q: What does an atom look like? l An atom is mostly empty space l Atoms have a small, dense nucleus at their center l Atoms contain what we call subatomic particles (3 subatomic particles)

Q: What does an atom look like? (3 subatomic particles) l Electron ¡ ¡ ¡ (symbol e-) Location- electron cloud (outside nucleus) Charge- negative (each electron has a -1 charge) Relative mass- 1/1840 l 1/1840 the size of the other particles = SMALL

Q: What does an atom look like? (3 subatomic particles) l Proton ¡ ¡ ¡ (symbol p+) Location- nucleus Charge- positive (each proton has a +1 charge) Relative mass- 1

Q: What does an atom look like? (3 subatomic particles) l Nucleus ¡ ¡ ¡ (symbol n 0) Location- nucleus Charge- neutral (each neutron has zero charge, not charged at all!) Relative mass- 1

Draw a picture of an atom below. Label each of the three subatomic particles, as well as the nucleus and electron cloud.

Q: What is the overall charge of the nucleus in an atom? Why? l Nucleus is made of protons and neutrons (p+ and n 0) l Since the only charged particles are protons and they have a positive charge, the overall charge is positive

Break Time l Structure of the Atom Worksheet (Chem. Quest 8) with questions ¡ Use the diagrams and information on the front of the sheet to answer the questions on the back.

Q: What is the Periodic Table? l Where ¡ we can look to find elements Any element in the world is found here. If it’s not here, it’s not an element. ¡ For example: Hydrogen and oxygen are found on the PT (H and O), they are both elements. l Water is made of these elements (H 2 O), but is not found on the PT. Water is a COMPOUND, not an element l

Q: What is the Periodic Table? l The table is organized based on physical and chemical properties/characteristics of elements such as: ¡ What will happen when it is mixed with a certain chemical? ¡ How many of each subatomic particle are present? ¡ What state of matter is it at room temperature? ¡ What does it look like (shiny, dull, etc)? ¡ How big is an atom of that element?

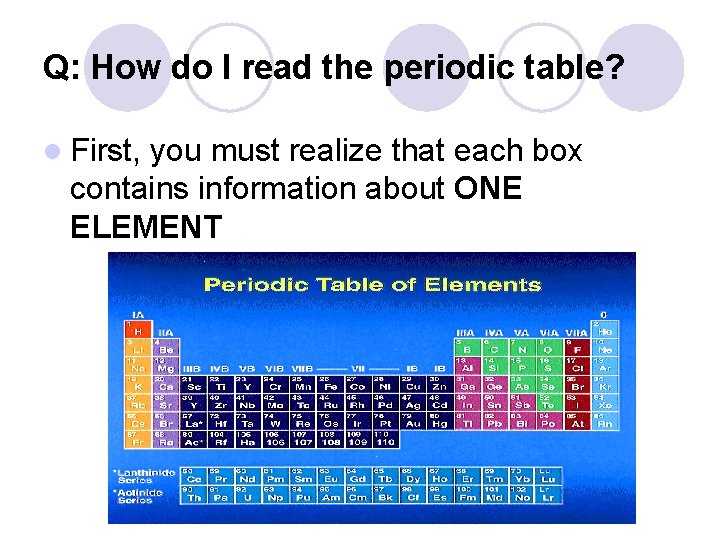
Q: How do I read the periodic table? l First, you must realize that each box contains information about ONE ELEMENT

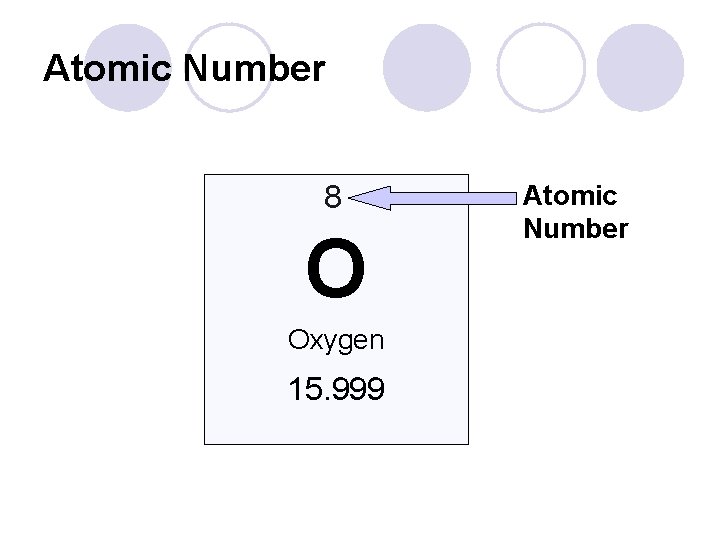
Q: How do I read the periodic table? l The smaller number in each box (usually at the top) is called the atomic number. ¡ Tells us how many p+ an atom of that element has ¡ Tells us how many e- an atom of that element has l *** This is true ONLY if the atom has an overall NEUTRAL CHARGE

Atomic Number 8 O Oxygen 15. 999 Atomic Number

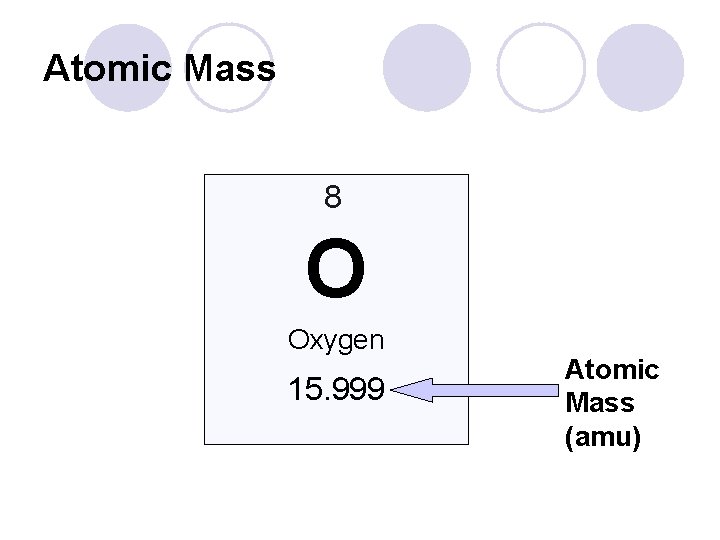
Q: How do I read the periodic table? l The larger number (usually at the bottom) represents the atomic mass. Measured in amu (atomic mass units) ¡ Tells us the total number of p+ and n 0 in an atom ¡ How can I use this to find JUST the number of n 0? ¡ Atomic mass – Atomic number = # n 0 l Atomic mass MUST be rounded to the nearest whole number l

Atomic Mass 8 O Oxygen 15. 999 Atomic Mass (amu)

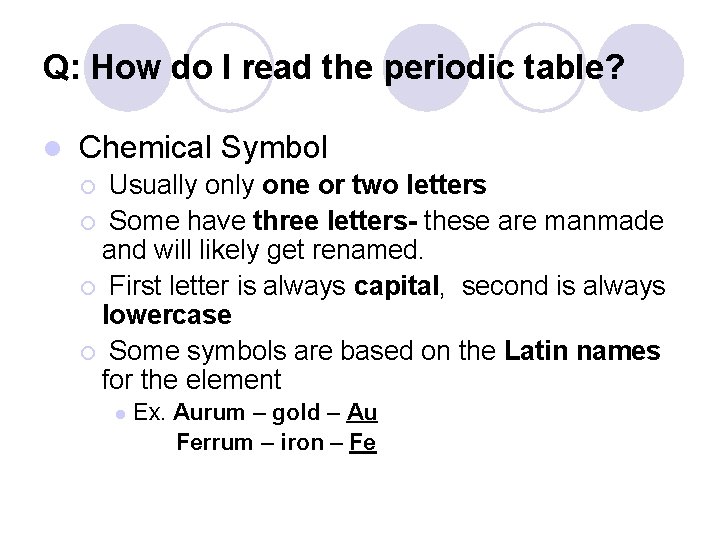
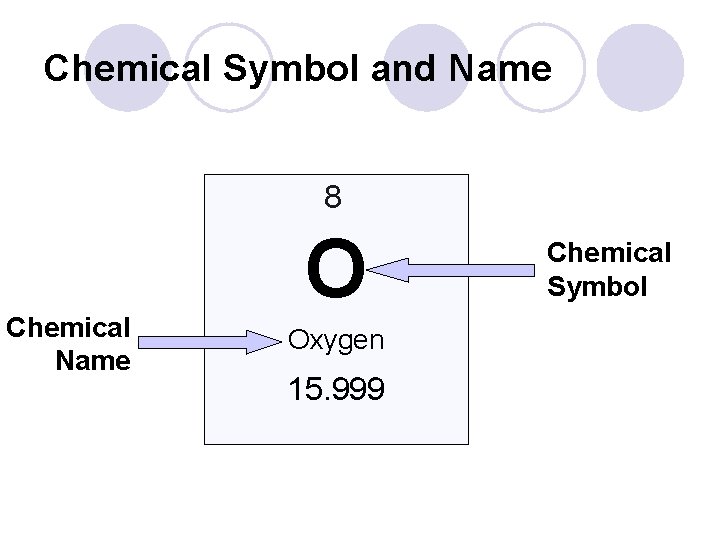
Q: How do I read the periodic table? l Chemical Symbol Usually one or two letters ¡ Some have three letters- these are manmade and will likely get renamed. ¡ First letter is always capital, second is always lowercase ¡ Some symbols are based on the Latin names for the element ¡ l Ex. Aurum – gold – Au Ferrum – iron – Fe

Chemical Symbol and Name 8 Chemical Name O Oxygen 15. 999 Chemical Symbol

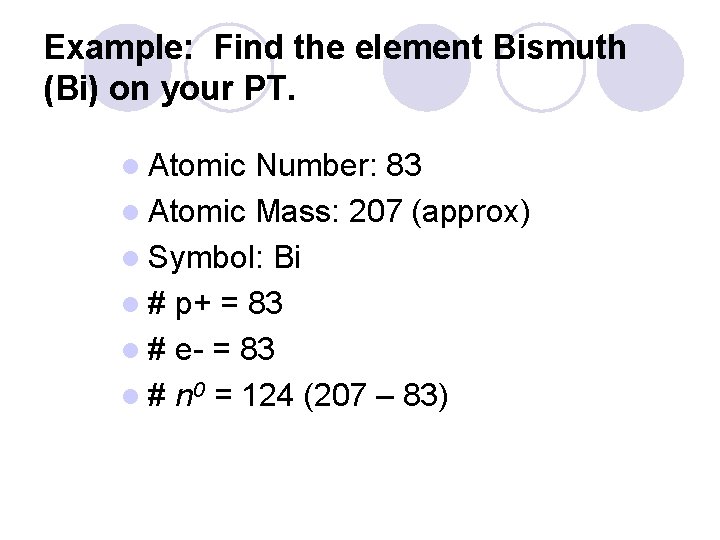
Example: Find the element Bismuth (Bi) on your PT. l Atomic Number: 83 l Atomic Mass: 207 (approx) l Symbol: Bi l # p+ = 83 l # e- = 83 l # n 0 = 124 (207 – 83)

Agenda Items l Atomic Math Challenge- due tomorrow