STRUCTURE OF THE ATOM The nucleus is the



- Slides: 9

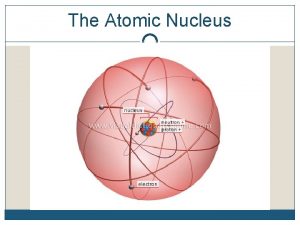
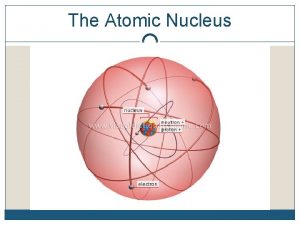
STRUCTURE OF THE ATOM • The nucleus is the “core” of the atom. • 99. 9 % of the mass of the atom is in the nucleus. – only protons and neutrons are found in the nucleus of an atom

PROTONS • PROTONS are positively charged particles found in the nucleus of an atom. • An element’s ATOMIC NUMBER tells the number of protons in an atom of that element’s nucleus. – (all hydrogen atoms have 1 proton and an atomic number of 1)

NEUTRONS • NEUTRONS are found in the nucleus of an atom, but they HAVE NO CHARGE! • Atoms of the same element that have the same number of protons but different numbers of neutrons are called ISOTOPES.

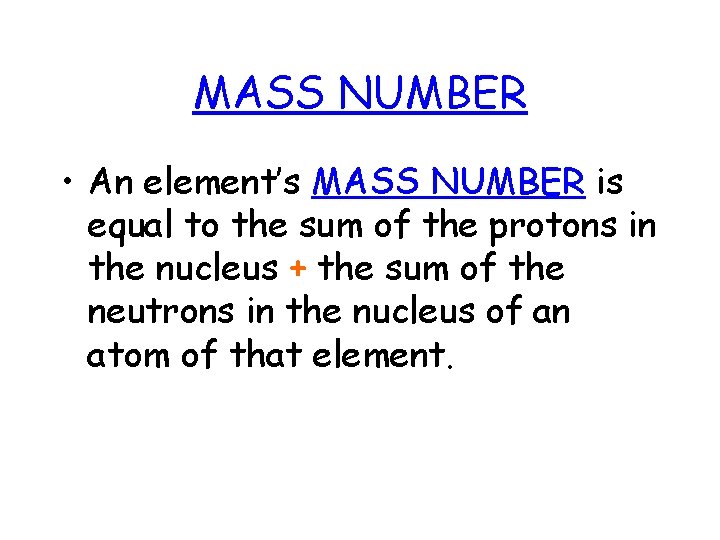
MASS NUMBER • An element’s MASS NUMBER is equal to the sum of the protons in the nucleus + the sum of the neutrons in the nucleus of an atom of that element.

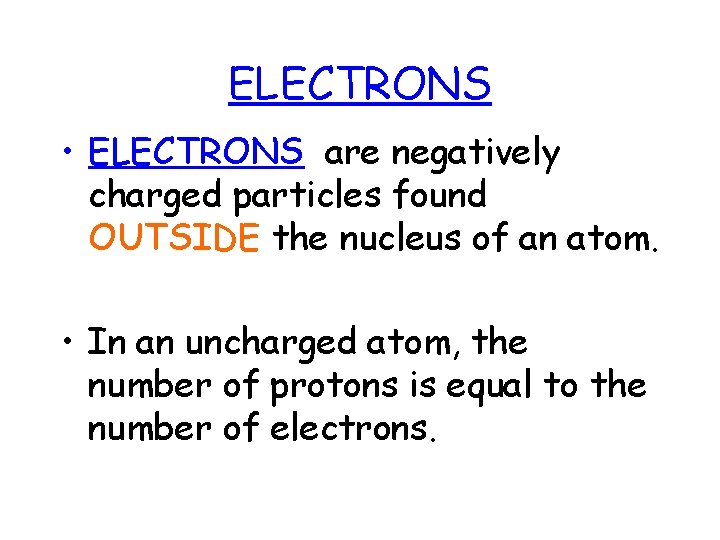
ELECTRONS • ELECTRONS are negatively charged particles found OUTSIDE the nucleus of an atom. • In an uncharged atom, the number of protons is equal to the number of electrons.

• The exact location of an electron is not known, but the probability of finding one in a given spot can be determined. • ENERGY LEVELS represent the most likely location in which an electron can be found.

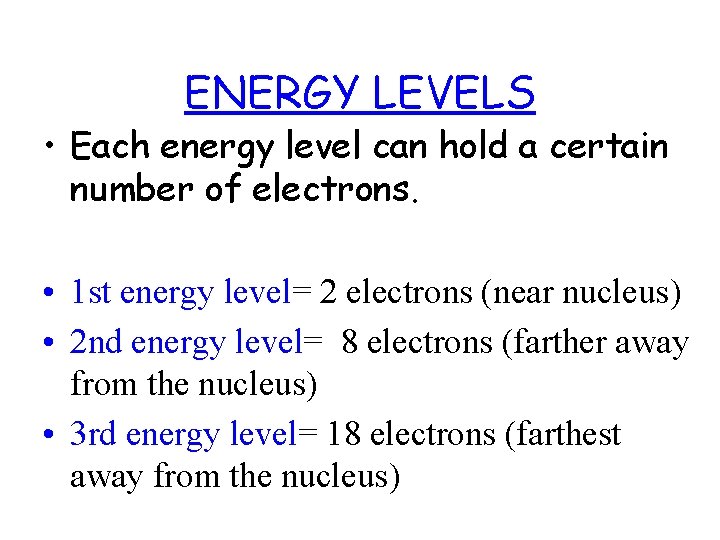
ENERGY LEVELS • Each energy level can hold a certain number of electrons. • 1 st energy level= 2 electrons (near nucleus) • 2 nd energy level= 8 electrons (farther away from the nucleus) • 3 rd energy level= 18 electrons (farthest away from the nucleus)

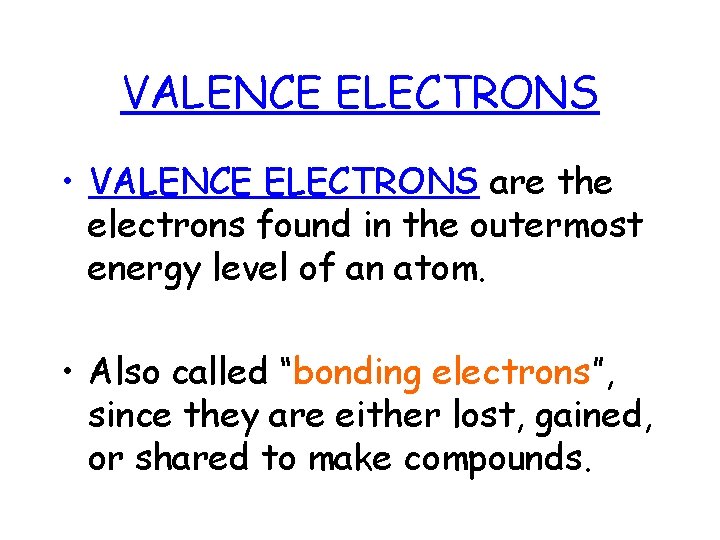
VALENCE ELECTRONS • VALENCE ELECTRONS are the electrons found in the outermost energy level of an atom. • Also called “bonding electrons”, since they are either lost, gained, or shared to make compounds.

• http: //www. brainpop. com/science/matterand chemistry/atoms