Classifying Matter ATOM Atom the smallest unit of















- Slides: 18

Classifying Matter

ATOM • Atom – the smallest unit of an element that maintains the properties of that element.

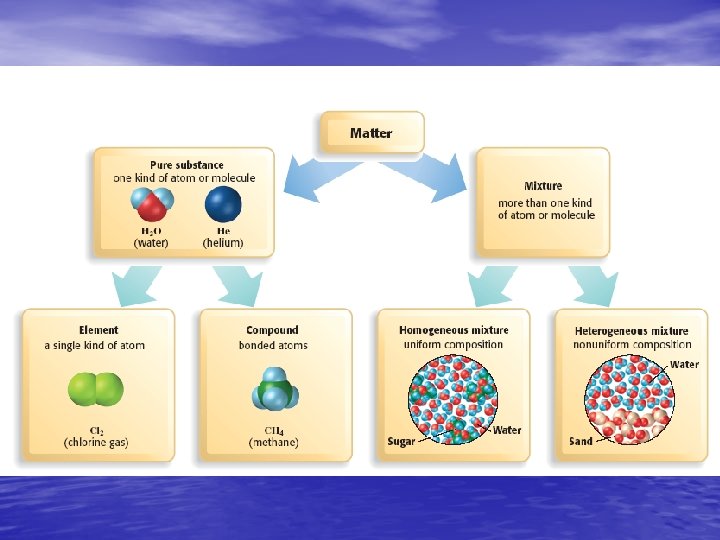
Pure Substance • A sample of matter, either a single element or a single compound, that has definite chemical and physical properties Figure 14, Page 22

Elements • A pure substance that contains only one kind of atom • All atoms of the same element have the same atomic number

Compounds • A pure substance that is made up of two or more different elements joined by chemical bonds.

Molecules • The smallest unit of a substance that keeps all of the physical and chemical properties of that substance.

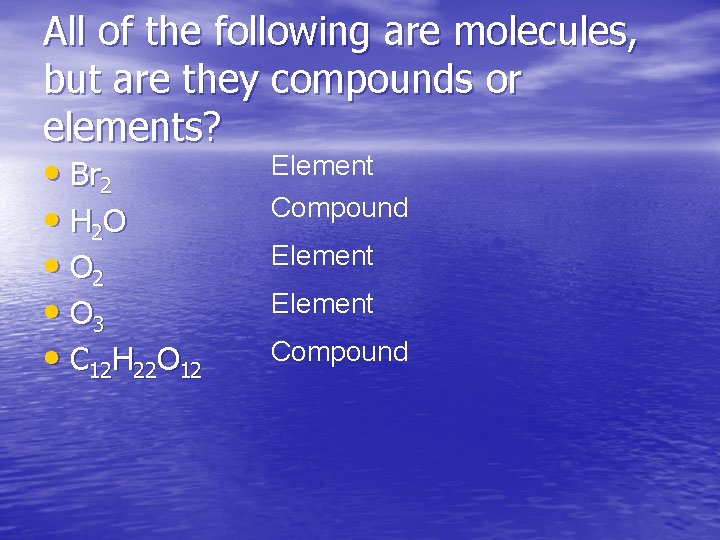
All of the following are molecules, but are they compounds or elements? Element • Br 2 Compound • H 2 O Element • O 2 Element • O 3 Compound • C 12 H 22 O 12

Mixtures • A combination of two or more substances that are not chemically combined. • Examples are air, ice tea, and even cake batter • The proportions of the substances can vary

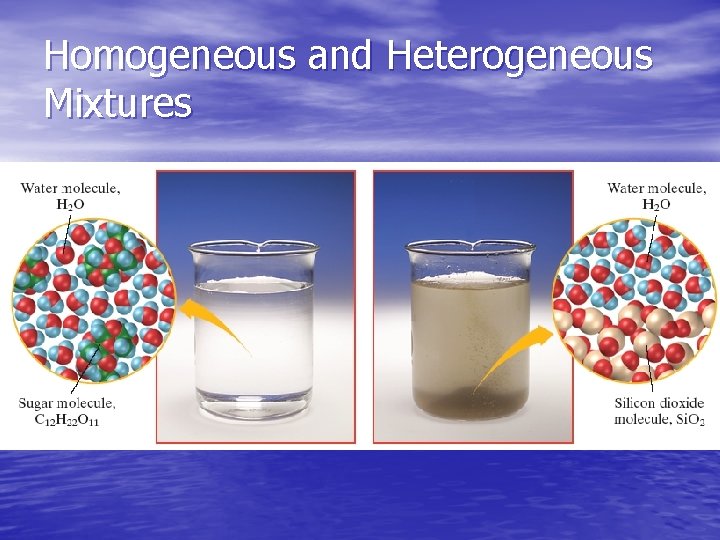
Homogeneous Mixtures • have a uniform structure or composition throughout • any two samples taken will have the same proportions of ingredients • Examples: Gasoline, air, and syrup

Heterogeneous Mixtures • NOT evenly mixed. • Different regions will have different proportions • Examples: Pulpy Orange Juice, chocolate chip cookie dough, and granite.

Homogeneous and Heterogeneous Mixtures


Separating Mixtures Since mixtures are just physically combined, they can be separated.

Separating Mixtures • Some Methods include: – Filtering – separation of a mixture’s components through differences in particle size – Decanting – a fancy term for separating two components by pouring – Distillation – used to separate two liquids based on their differences in boiling points – Magnetism – used to separate magnetic substances – Evaporation – removing a liquid to leave a solid behind – Centrifuge – separates substances of different densities using a fast rotational motion – Chromatography – Separates two substances by using a mobile phase and a stationary phase

Physical Changes • A change of matter from one form to another without changing the substance itself. • A A • Examples: phase changes, mixtures

Chemical Changes • when one or more substances change into entirely new substances with different properties • A + B C (reactants go to products)

Release or An Unexpected Formation of a Absorption Formation of Color Precipitate of Energy a gas Change (solid)

Chemical or Physical • Frying an egg - Chemical • Boiling Water - Physical • Sanding a wooden plank - Physical • Digesting food - Chemical • Popping a balloon - Physical