Representative Particles Whatever type of particle that is


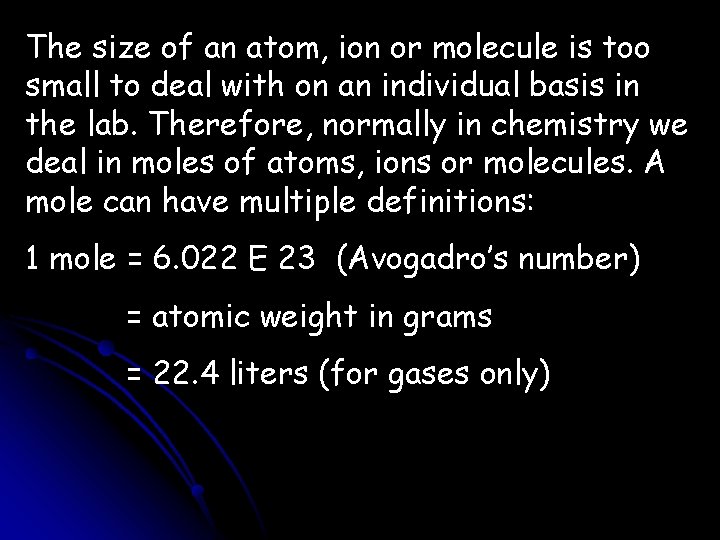


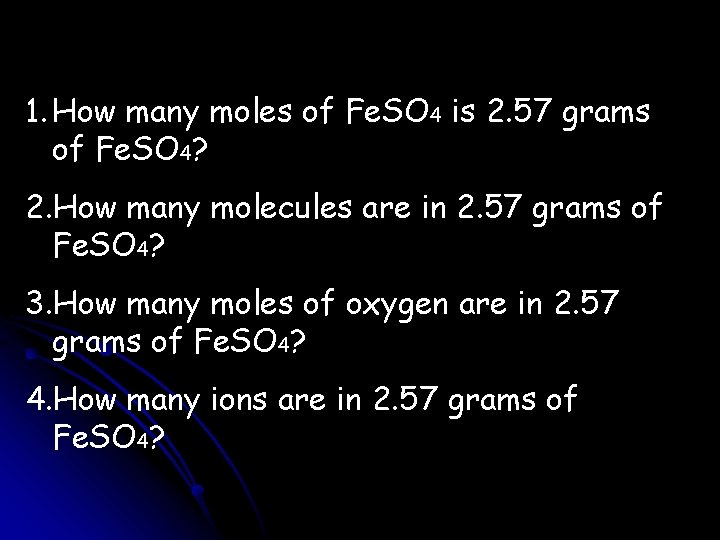


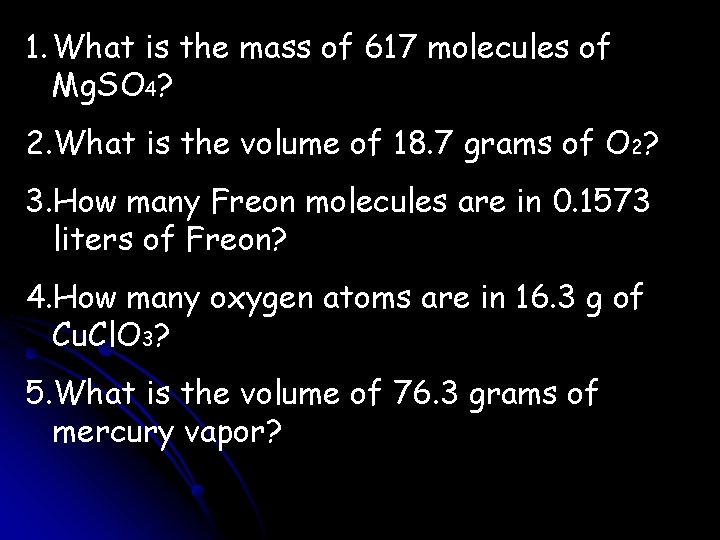
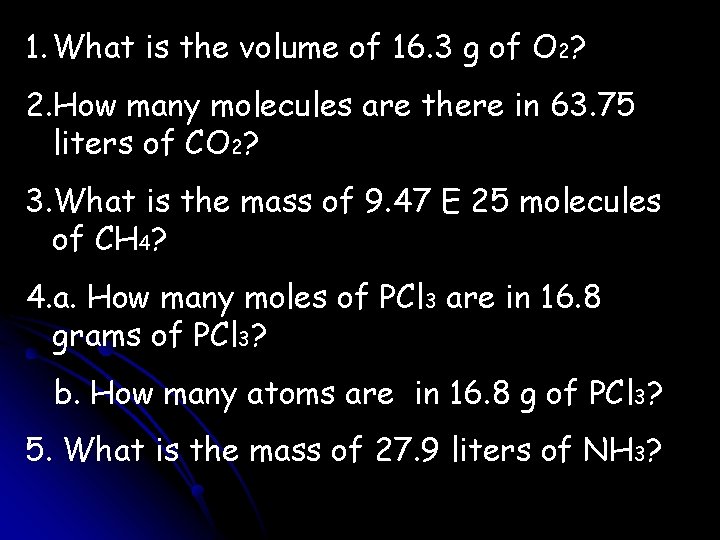
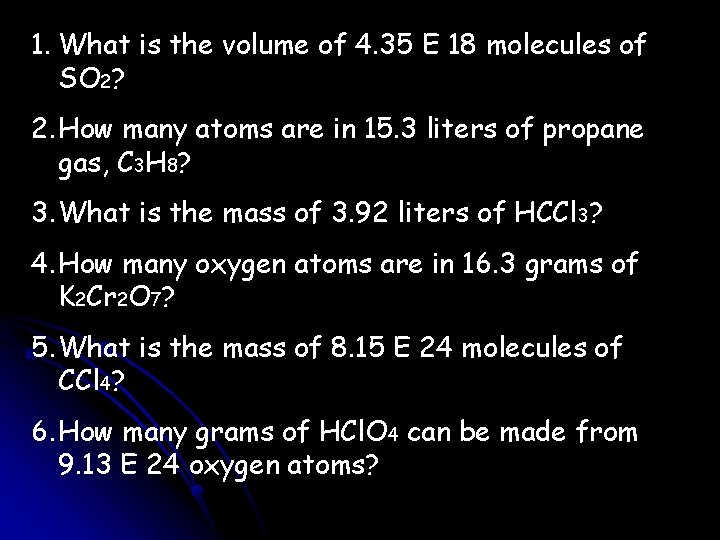

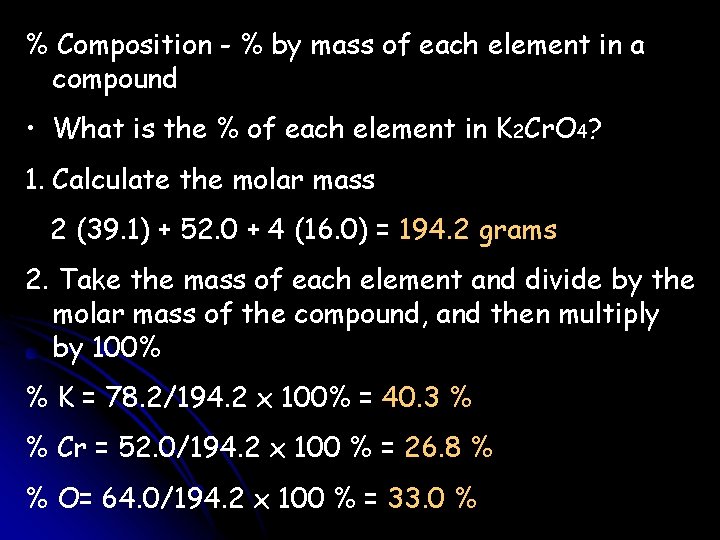

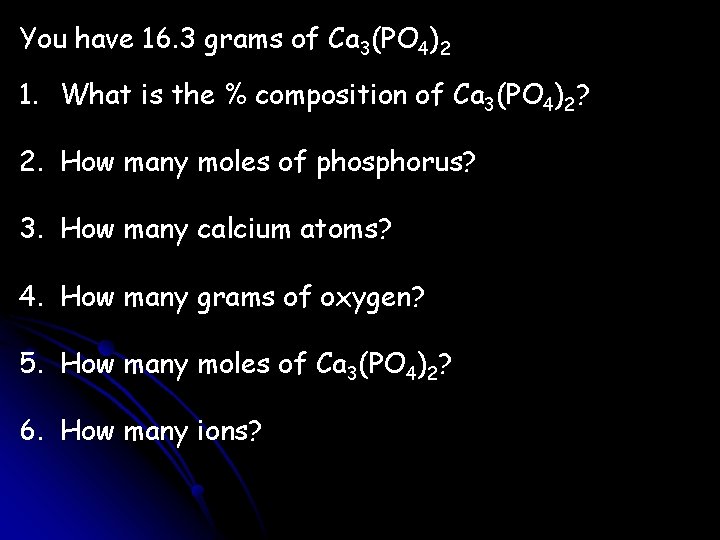

- Slides: 25

Representative Particles Whatever type of particle that is being examined. • Atom – any single element that is part of the overall compound. • Ion – an atom or group of atoms that has a net charge • Molecule – a group of atoms that have been chemically combined. There is no net charge on a molecule.

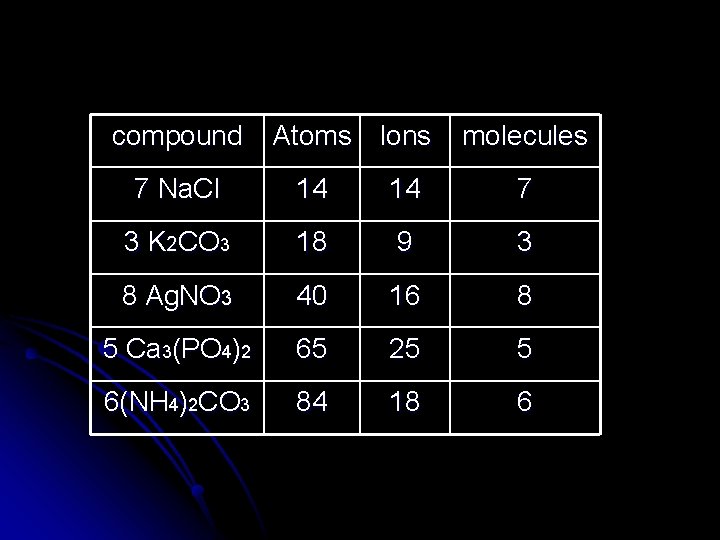
Determine how many atoms, ions and molecules are in each example: Pb(NO 3)2 1 molecule 3 ions ( 1 Pb+2, 2 NO 3 -1) 9 atoms ( 1 Pb, 2 N, 6 O) 5 (NH 4)2 SO 4 5 molecules 15 ions ( 10 NH 4+1, 5 SO 4 -2) 75 atoms ( 10 N, 40 H, 5 S, 20 O)

compound Atoms Ions molecules 7 Na. Cl 14 14 7 3 K 2 CO 3 18 9 3 8 Ag. NO 3 40 16 8 5 Ca 3(PO 4)2 65 25 5 6(NH 4)2 CO 3 84 18 6
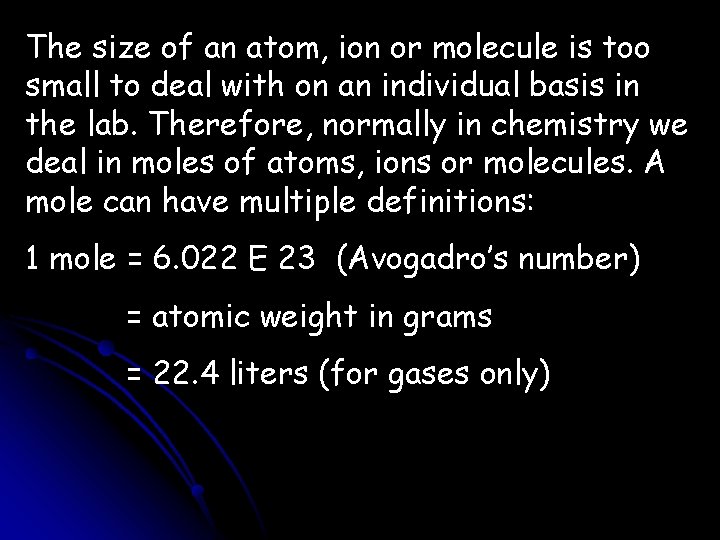
The size of an atom, ion or molecule is too small to deal with on an individual basis in the lab. Therefore, normally in chemistry we deal in moles of atoms, ions or molecules. A mole can have multiple definitions: 1 mole = 6. 022 E 23 (Avogadro’s number) = atomic weight in grams = 22. 4 liters (for gases only)

Molar Mass – the mass, in grams, of 1 mole of any substance. It is found by taking the atomic weight from the periodic table, and rounding to one place past the decimal. The molar mass of each of the following: Be = 9. 0 grams Cr = 52. 0 grams Cl = 35. 5 grams S = 32. 1 grams O = 16. 0 grams

Calculate the molar mass of the following: Be. Cl 2, Be. SO 4, Cr(Cl. O 3)3, Cr 2(SO 4)3 and (NH 4)3 PO 4 Be. Cl 2 : 9. 0 + 2 (35. 5) = 80. 0 grams Be. SO 4 : 9. 0 + 32. 1 + 4 (16. 0) = 105. 1 grams Cr(Cl. O 3)3 : 52. 0 + 3 (35. 5) + 9 (16. 0) = 302. 5 grams Cr 2(SO 4)3 : 2 (52. 0) + 3 (32. 1) + 12 (16. 0) = 392. 3 grams (NH 4)3 PO 4 : 3 (14. 0) + 12 (1. 0) + 31. 0 + 4 (16. 0) = 149. 0 g

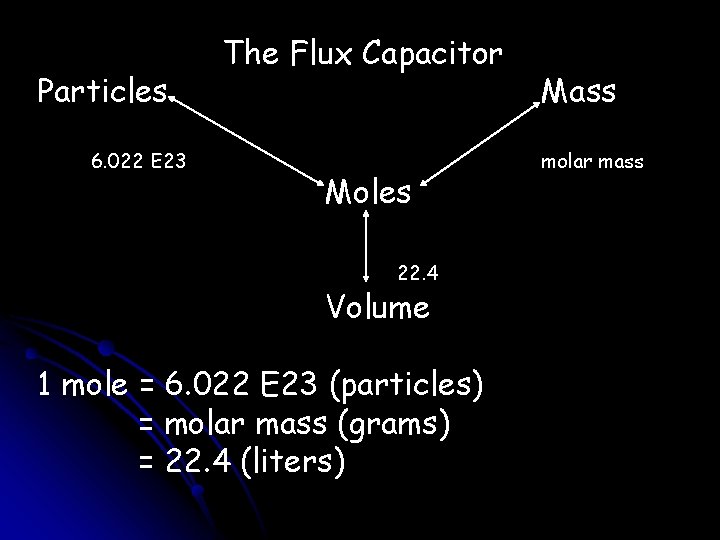
Particles 6. 022 E 23 The Flux Capacitor Moles 22. 4 Volume 1 mole = 6. 022 E 23 (particles) = molar mass (grams) = 22. 4 (liters) Mass molar mass

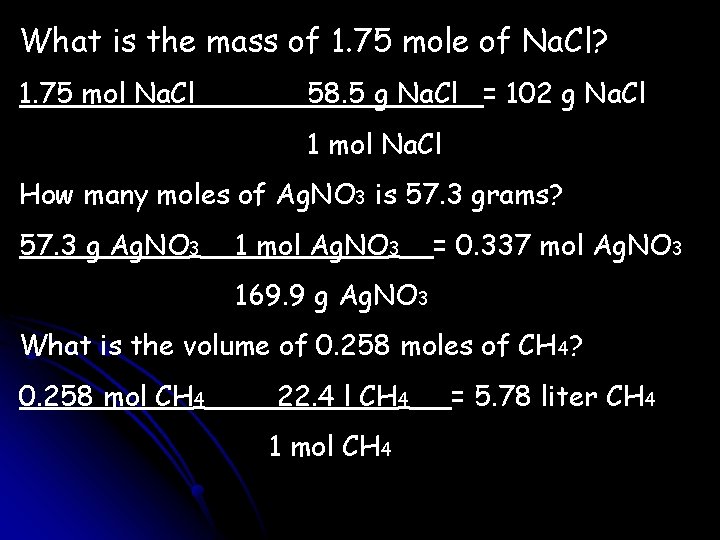
What is the mass of 1. 75 mole of Na. Cl? 1. 75 mol Na. Cl 58. 5 g Na. Cl = 102 g Na. Cl 1 mol Na. Cl How many moles of Ag. NO 3 is 57. 3 grams? 57. 3 g Ag. NO 3 1 mol Ag. NO 3 = 0. 337 mol Ag. NO 3 169. 9 g Ag. NO 3 What is the volume of 0. 258 moles of CH 4? 0. 258 mol CH 4 22. 4 l CH 4 1 mol CH 4 = 5. 78 liter CH 4
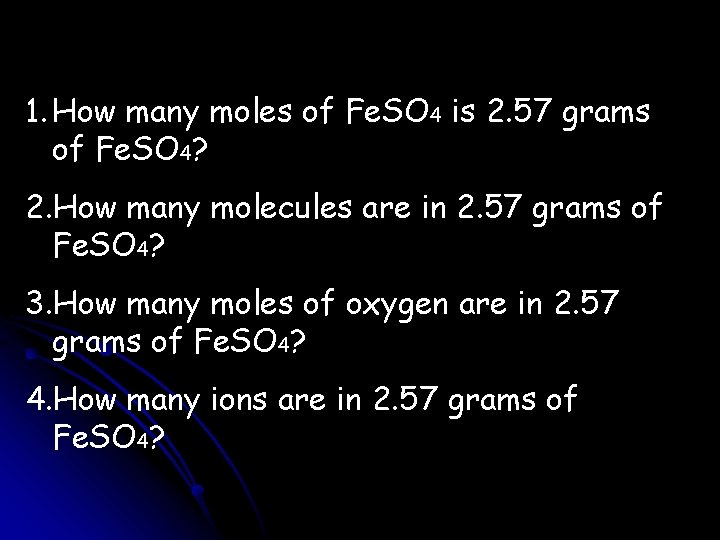
1. How many moles of Fe. SO 4 is 2. 57 grams of Fe. SO 4? 2. How many molecules are in 2. 57 grams of Fe. SO 4? 3. How many moles of oxygen are in 2. 57 grams of Fe. SO 4? 4. How many ions are in 2. 57 grams of Fe. SO 4?

1. 2. 57 g Fe. SO 4 1 mol Fe. SO 4 = 0. 0169 mol Fe. SO 4 151. 9 g Fe. SO 4 2. 2. 57 g Fe. SO 4 1 mol Fe. SO 4 6. 022 E 23 molc Fe. SO 4 = 151. 9 g Fe. SO 4 1 mol Fe. SO 4 1. 02 E 22 molc Fe. SO 4 3. 2. 57 g Fe. SO 4 1 mol Fe. SO 4 151. 9 g Fe. SO 4 4. 2. 57 g Fe. SO 4 1 mol Fe. SO 4 4 mol O = 0. 0677 mol O 1 mol Fe. SO 4 6. 022 E 23 molc 151. 9 g Fe. SO 4 1 mol Fe. SO 4 2 ions = 1 molc 2. 04 E 22 ions

How many atoms are in 17. 3 grams of Fe 2 O 3? 17. 3 g Fe 2 O 3 1 mol Fe 2 O 3 159. 6 g Fe 2 O 3 6. 02 E 23 Fe 2 O 3 5 atoms = 1 mol Fe 2 O 3 1 Fe 2 O 3 3. 26 E 23 atoms What is the mass of 1. 16 E 21 molecules of Fe 2 O 3? 1. 16 E 21 molc Fe 2 O 3 1 mol Fe 2 O 3 159. 6 g Fe 2 O 3 = 6. 02 E 23 molc Fe 2 O 3 1 mol Fe 2 O 3 0. 307 g Fe 2 O 3
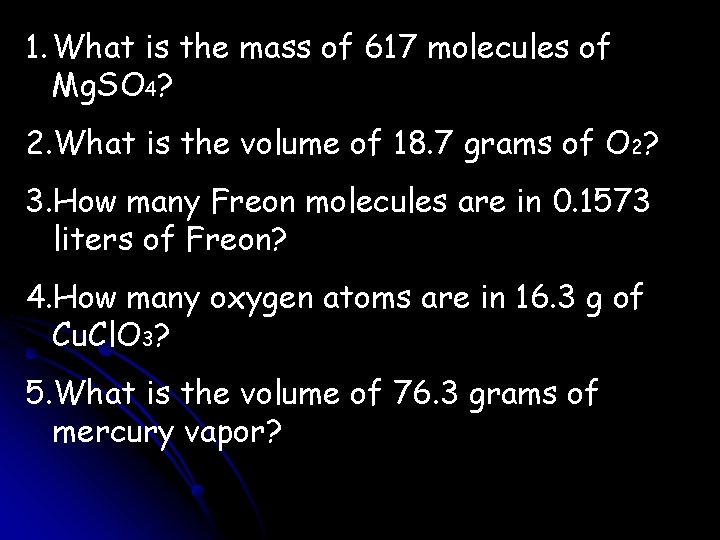
1. What is the mass of 617 molecules of Mg. SO 4? 2. What is the volume of 18. 7 grams of O 2? 3. How many Freon molecules are in 0. 1573 liters of Freon? 4. How many oxygen atoms are in 16. 3 g of Cu. Cl. O 3? 5. What is the volume of 76. 3 grams of mercury vapor?
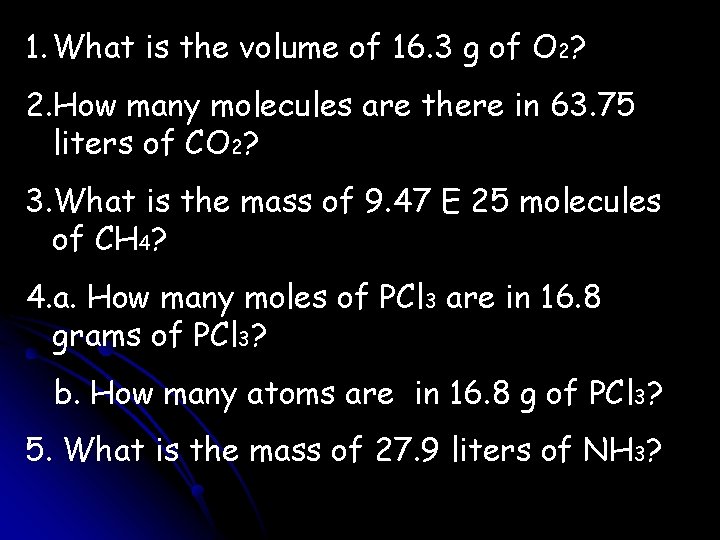
1. What is the volume of 16. 3 g of O 2? 2. How many molecules are there in 63. 75 liters of CO 2? 3. What is the mass of 9. 47 E 25 molecules of CH 4? 4. a. How many moles of PCl 3 are in 16. 8 grams of PCl 3? b. How many atoms are in 16. 8 g of PCl 3? 5. What is the mass of 27. 9 liters of NH 3?
1. What is the volume of 4. 35 E 18 molecules of SO 2? 2. How many atoms are in 15. 3 liters of propane gas, C 3 H 8? 3. What is the mass of 3. 92 liters of HCCl 3? 4. How many oxygen atoms are in 16. 3 grams of K 2 Cr 2 O 7? 5. What is the mass of 8. 15 E 24 molecules of CCl 4? 6. How many grams of HCl. O 4 can be made from 9. 13 E 24 oxygen atoms?

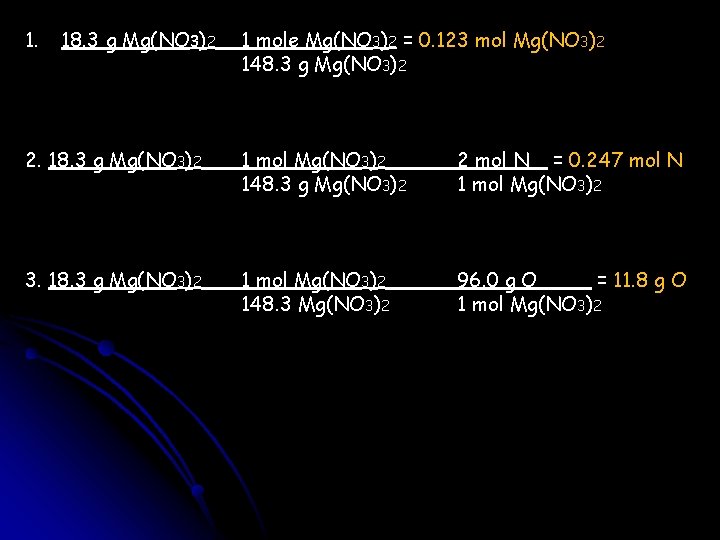
You are starting with 18. 3 g of Mg(NO 3)2. 1. How many moles of Mg(NO 3)2 are there? 2. How many moles of N are there? 3. How many grams of oxygen are present?

1. 18. 3 g Mg(NO 3)2 1 mole Mg(NO 3)2 = 0. 123 mol Mg(NO 3)2 148. 3 g Mg(NO 3)2 2. 18. 3 g Mg(NO 3)2 1 mol Mg(NO 3)2 148. 3 g Mg(NO 3)2 2 mol N = 0. 247 mol N 1 mol Mg(NO 3)2 3. 18. 3 g Mg(NO 3)2 1 mol Mg(NO 3)2 148. 3 Mg(NO 3)2 96. 0 g O = 11. 8 g O 1 mol Mg(NO 3)2
% Composition - % by mass of each element in a compound • What is the % of each element in K 2 Cr. O 4? 1. Calculate the molar mass 2 (39. 1) + 52. 0 + 4 (16. 0) = 194. 2 grams 2. Take the mass of each element and divide by the molar mass of the compound, and then multiply by 100% % K = 78. 2/194. 2 x 100% = 40. 3 % % Cr = 52. 0/194. 2 x 100 % = 26. 8 % % O= 64. 0/194. 2 x 100 % = 33. 0 %

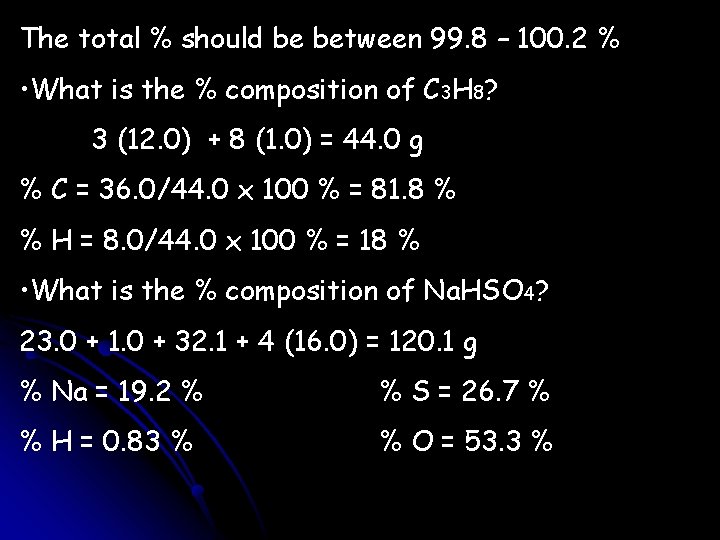
The total % should be between 99. 8 – 100. 2 % • What is the % composition of C 3 H 8? 3 (12. 0) + 8 (1. 0) = 44. 0 g % C = 36. 0/44. 0 x 100 % = 81. 8 % % H = 8. 0/44. 0 x 100 % = 18 % • What is the % composition of Na. HSO 4? 23. 0 + 1. 0 + 32. 1 + 4 (16. 0) = 120. 1 g % Na = 19. 2 % % S = 26. 7 % % H = 0. 83 % % O = 53. 3 %

How many grams of sodium are in 4. 76 grams of Na. HSO 4? 4. 76 g Na. HSO 4 23. 0 g Na = 0. 912 g Na 120. 1 g Na. HSO 4 How many grams of oxygen are in 4. 76 grams of Na. HSO 4? 4. 76 g Na. HSO 4 64. 0 g O 120. 1 g Na. HSO 4 = 2. 54 g O
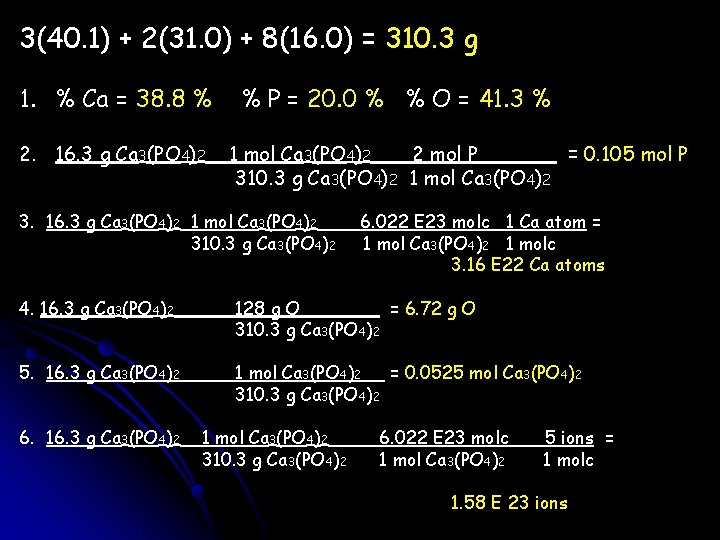
You have 16. 3 grams of Ca 3(PO 4)2 1. What is the % composition of Ca 3(PO 4)2? 2. How many moles of phosphorus? 3. How many calcium atoms? 4. How many grams of oxygen? 5. How many moles of Ca 3(PO 4)2? 6. How many ions?

3(40. 1) + 2(31. 0) + 8(16. 0) = 310. 3 g 1. % Ca = 38. 8 % 2. 16. 3 g Ca 3(PO 4)2 % P = 20. 0 % % O = 41. 3 % 1 mol Ca 3(PO 4)2 2 mol P = 0. 105 mol P 310. 3 g Ca 3(PO 4)2 1 mol Ca 3(PO 4)2 3. 16. 3 g Ca 3(PO 4)2 1 mol Ca 3(PO 4)2 310. 3 g Ca 3(PO 4)2 6. 022 E 23 molc 1 Ca atom = 1 mol Ca 3(PO 4)2 1 molc 3. 16 E 22 Ca atoms 4. 16. 3 g Ca 3(PO 4)2 128 g O = 6. 72 g O 310. 3 g Ca 3(PO 4)2 5. 16. 3 g Ca 3(PO 4)2 1 mol Ca 3(PO 4)2 = 0. 0525 mol Ca 3(PO 4)2 310. 3 g Ca 3(PO 4)2 6. 16. 3 g Ca 3(PO 4)2 1 mol Ca 3(PO 4)2 310. 3 g Ca 3(PO 4)2 6. 022 E 23 molc 1 mol Ca 3(PO 4)2 5 ions = 1 molc 1. 58 E 23 ions

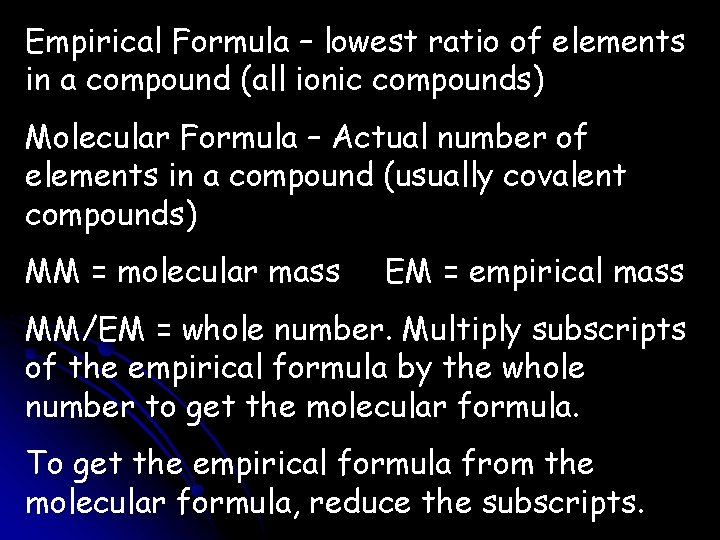
Empirical Formula – lowest ratio of elements in a compound (all ionic compounds) Molecular Formula – Actual number of elements in a compound (usually covalent compounds) MM = molecular mass EM = empirical mass MM/EM = whole number. Multiply subscripts of the empirical formula by the whole number to get the molecular formula. To get the empirical formula from the molecular formula, reduce the subscripts.

What is the molecular formula of a compound with a molecular mass of 60. 0 grams, and an empirical formula of CH 4 N? EM = 12. 0 + 4 (1. 0) + 14. 0 = 30. 0 grams MM/EM = 60. 0/30. 0 = 2 2 x (CH 4 N) = C 2 H 8 N 2

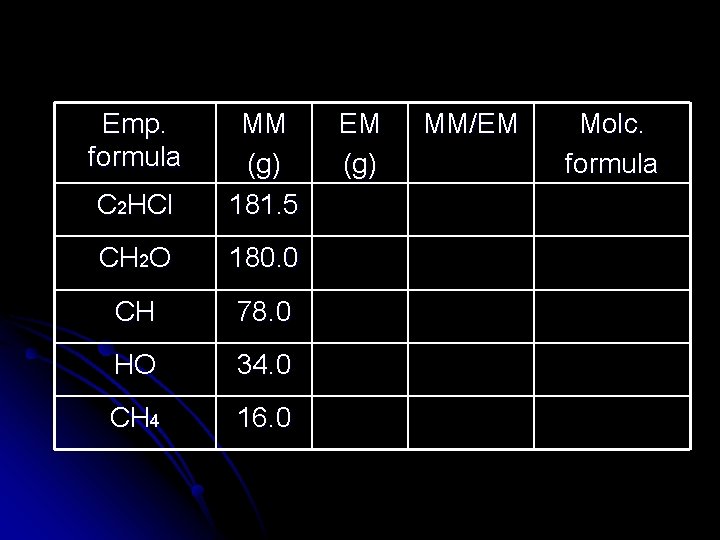
Emp. formula C 2 HCl MM (g) 181. 5 CH 2 O 180. 0 CH 78. 0 HO 34. 0 CH 4 16. 0 EM (g) MM/EM Molc. formula

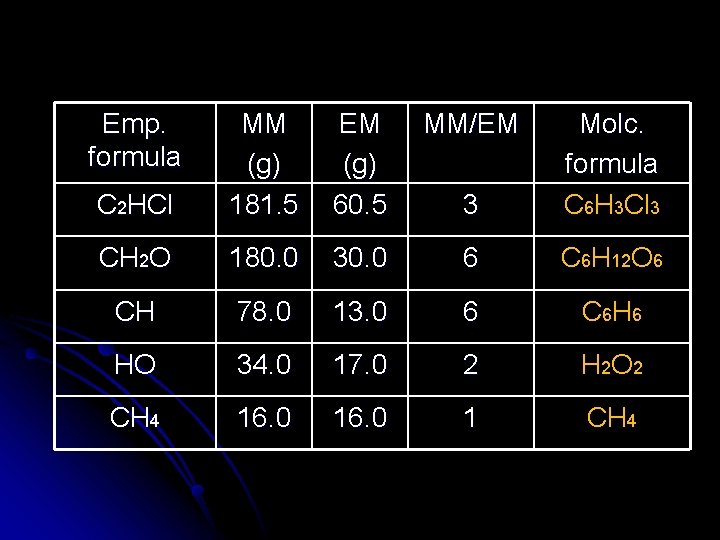
Emp. formula EM (g) 60. 5 MM/EM C 2 HCl MM (g) 181. 5 3 Molc. formula C 6 H 3 Cl 3 CH 2 O 180. 0 30. 0 6 C 6 H 12 O 6 CH 78. 0 13. 0 6 C 6 H 6 HO 34. 0 17. 0 2 H 2 O 2 CH 4 16. 0 1 CH 4