1 Atom The smallest particle of an element





- Slides: 16

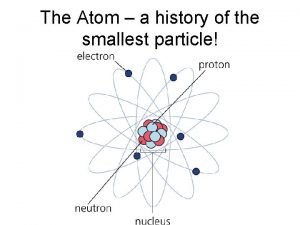
1. Atom • The smallest particle of an element that still has the properties of that element

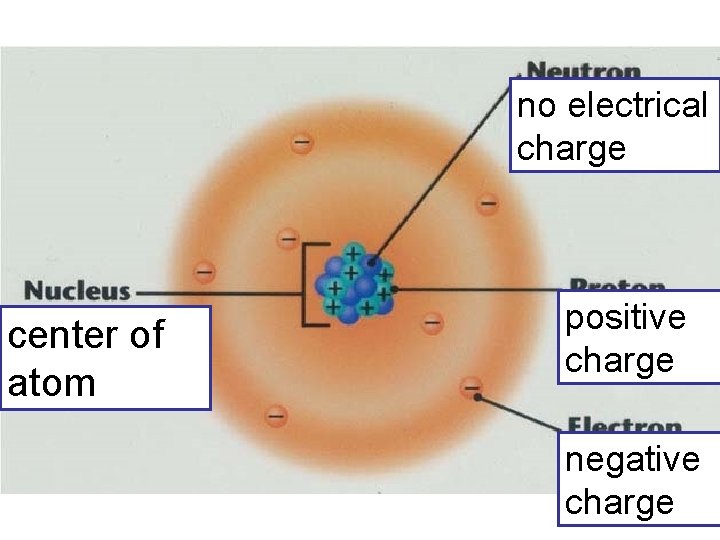
no electrical charge center of atom positive charge negative charge

2. Compound • Made of combination of elements • Properties of compounds often differ from properties of elements that make them up

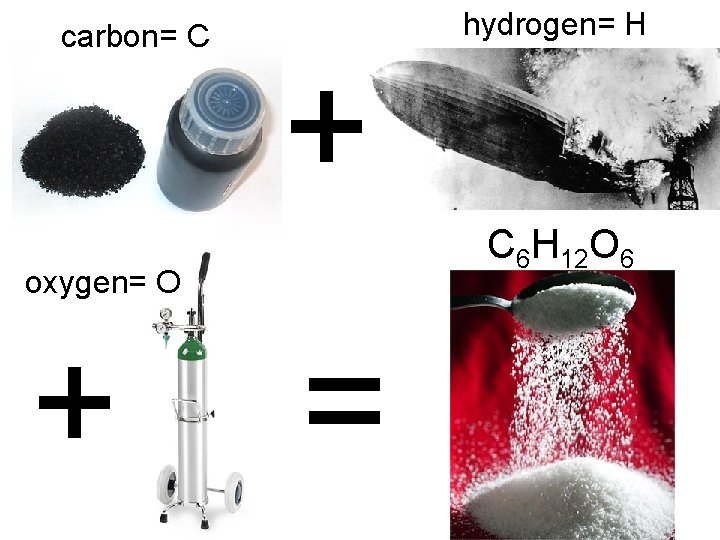
carbon= C hydrogen= H + C 6 H 12 O 6 oxygen= O + =

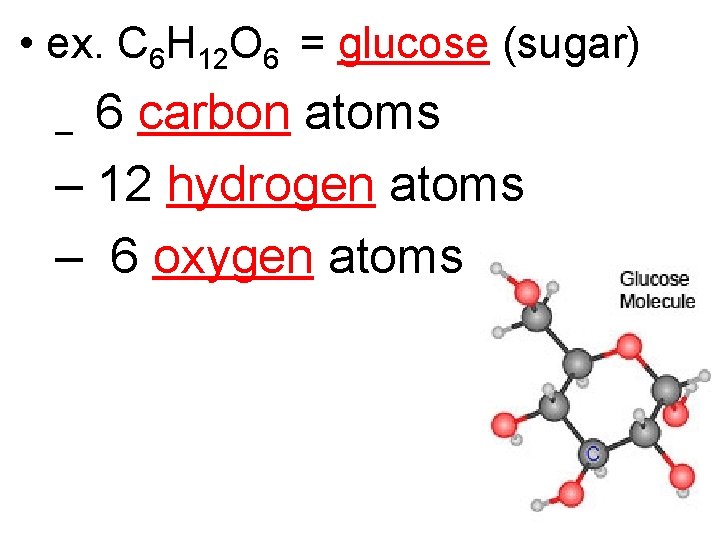
• ex. C 6 H 12 O 6 = glucose (sugar) 6 carbon atoms – 12 hydrogen atoms – 6 oxygen atoms –

3. Atomic Theory – the beginning • Democritus believed that the tiniest particle was the atom, which was the smallest thing that could ________________. • He also thought that atoms were made of a _________________ that formed into different shapes and sizes. be divided to single material Atomos indivisible

4. John Dalton’s Atomic Theory • Based on scientific _____________ through _________, John Dalton published a theory in 1803. His theory states these ideas: evidence experiments

4. John Dalton’s Atomic Theory • All substances are made of ________. Atoms are small particles that cannot be ________, _________, or _____________. • Atoms of the same element are ________________. and the atoms of different elements are ______________. • Atoms join with other atoms to make _______________. atoms created divided destroyed exactly alike different. new substances

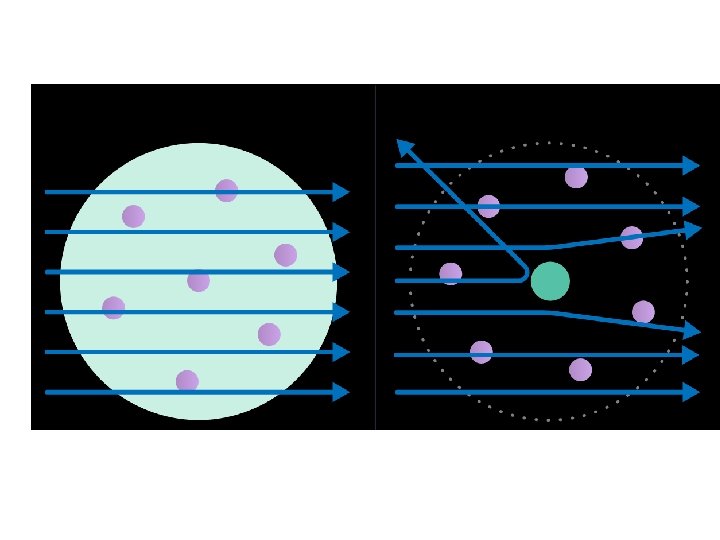
5. Disagreeing with Dalton • As scientists were able to gather new data, more discoveries about the atom showed some mistakes with Dalton’s theory. • • J. J. Thompson discovered with a _____________ that atoms contained __________-charged particles called __________. He came up with the _______________ model. cathode-ray negatively electrons. Plum-pudding

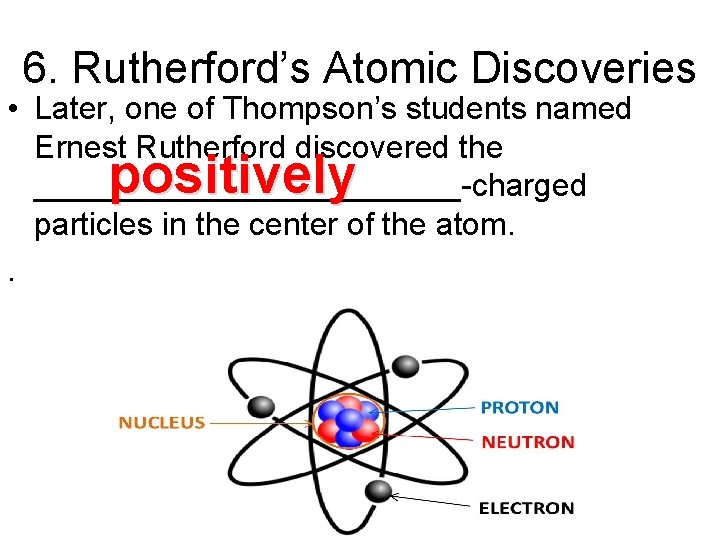
6. Rutherford’s Atomic Discoveries • Later, one of Thompson’s students named Ernest Rutherford discovered the ____________-charged particles in the center of the atom. . positively

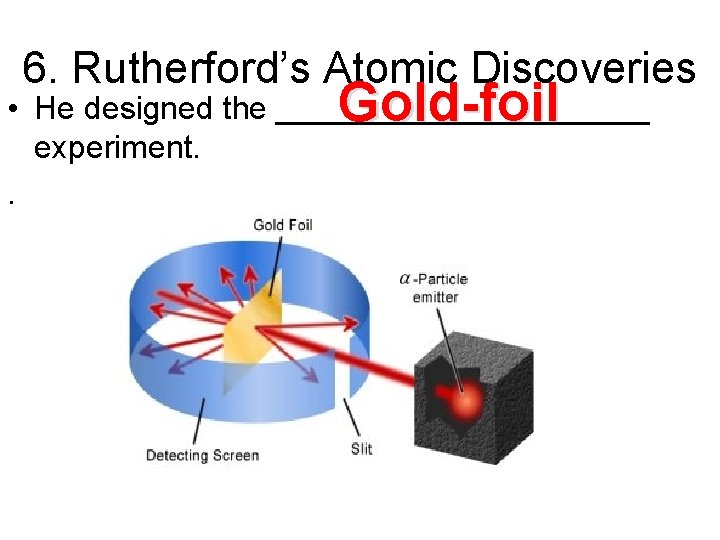
6. Rutherford’s Atomic Discoveries Gold-foil • He designed the ___________ experiment. .


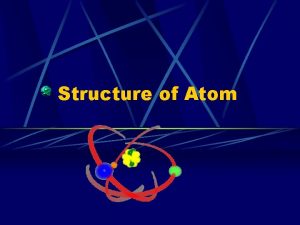
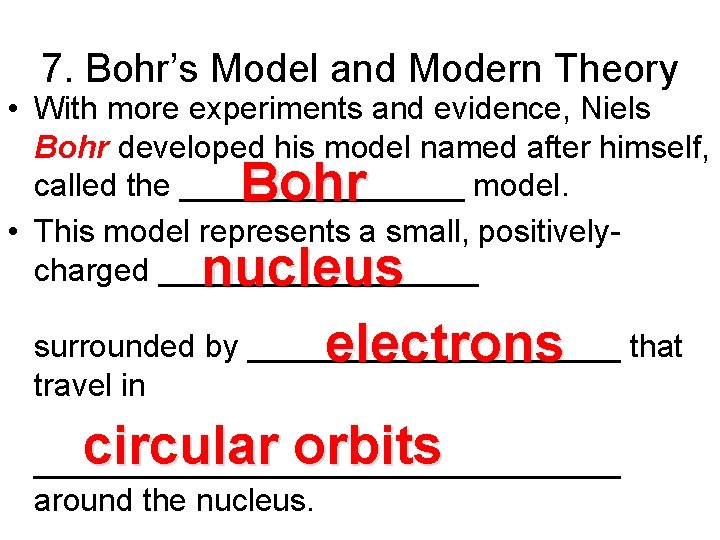
7. Bohr’s Model and Modern Theory • With more experiments and evidence, Niels Bohr developed his model named after himself, called the ________ model. • This model represents a small, positivelycharged _________ Bohr nucleus surrounded by ___________ electrons that travel in circular orbits _________________ around the nucleus.


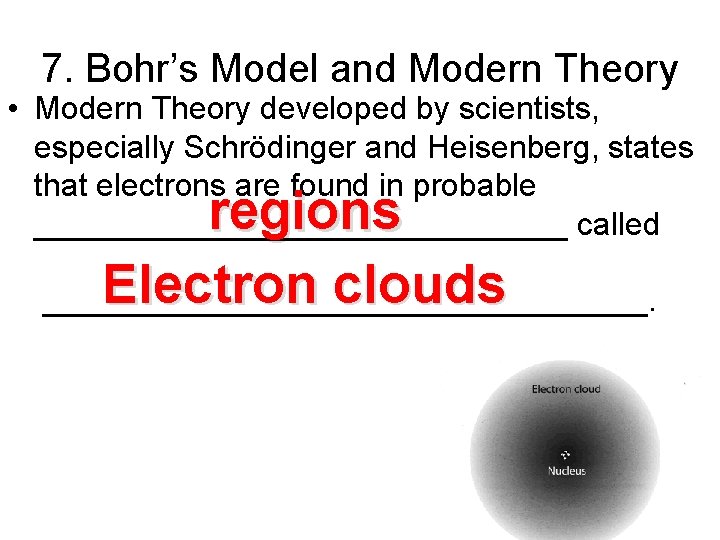
7. Bohr’s Model and Modern Theory • Modern Theory developed by scientists, especially Schrödinger and Heisenberg, states that electrons are found in probable _______________ called regions Electron clouds _________________.

Now let’s draw the models that evolved throughout history.
 The smallest particle of an element
The smallest particle of an element Whats the smallest particle of an element
Whats the smallest particle of an element Whats the smallest particle of matter
Whats the smallest particle of matter Smallest particle
Smallest particle Smallest particle on earth
Smallest particle on earth Smallest unit of charge is franklin
Smallest unit of charge is franklin Smallest known particle
Smallest known particle Particle atom molecule
Particle atom molecule Atom
Atom Solid element particle diagram
Solid element particle diagram Representative particle
Representative particle Example of representative particle
Example of representative particle Smallest part of an element
Smallest part of an element The smallest piece of an element
The smallest piece of an element Smallest part of a compound
Smallest part of a compound The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Kelemahan teori rutherford
Kelemahan teori rutherford