Chapter 7 n Representative particle refers to whether

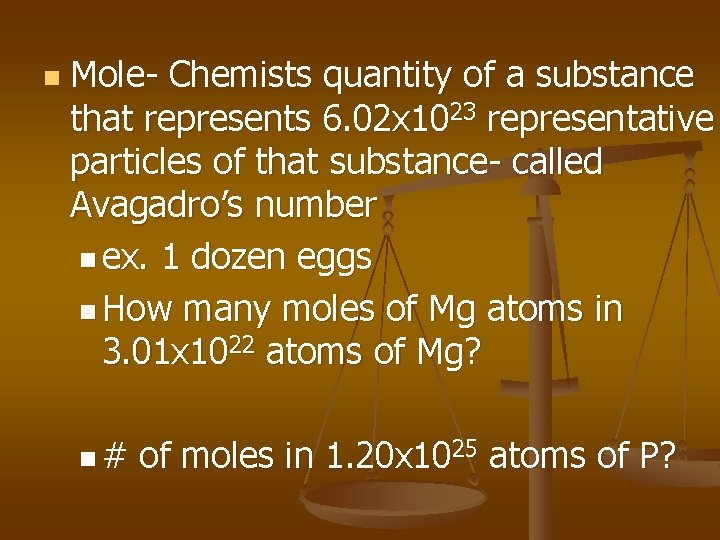
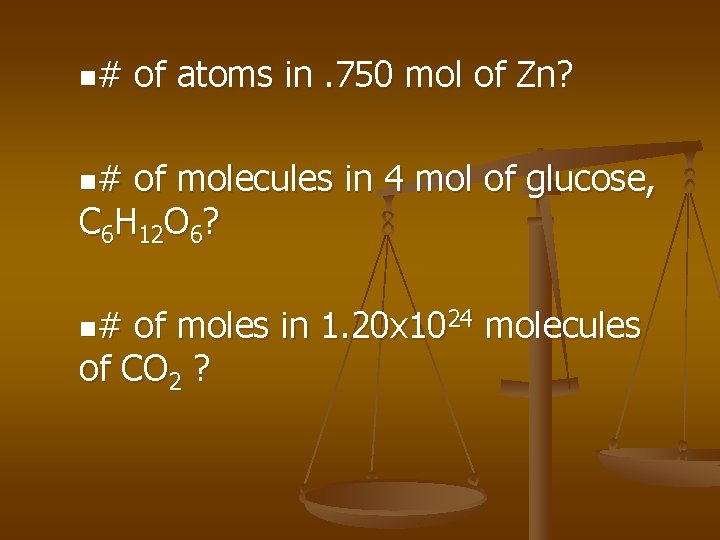






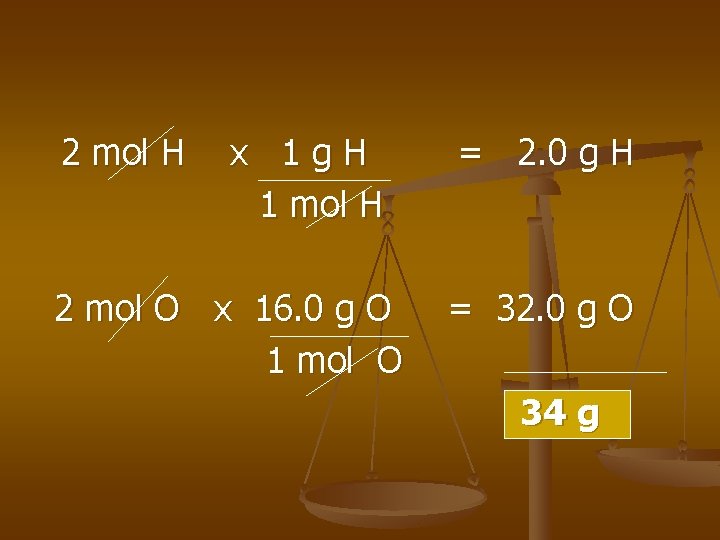

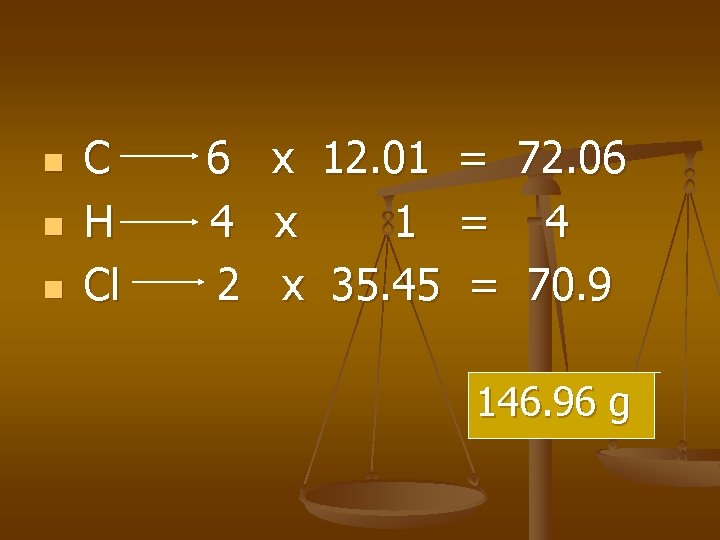


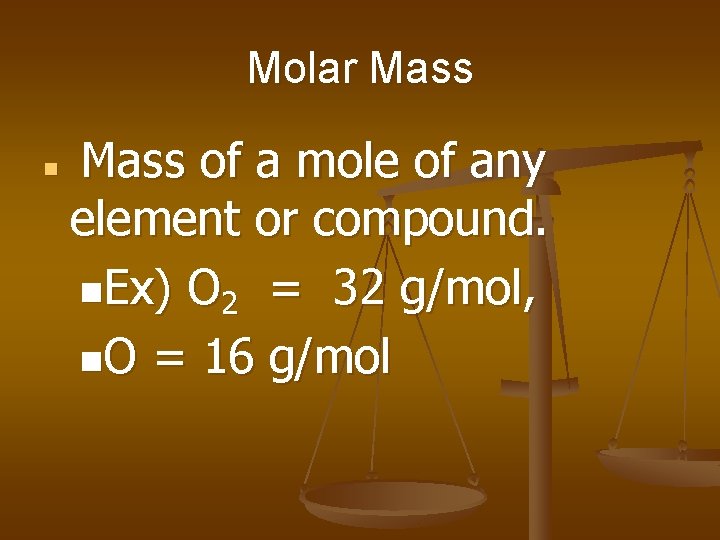

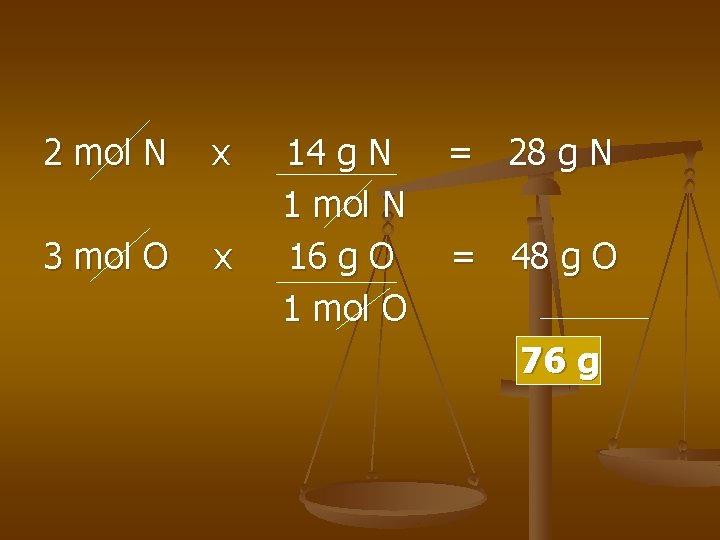

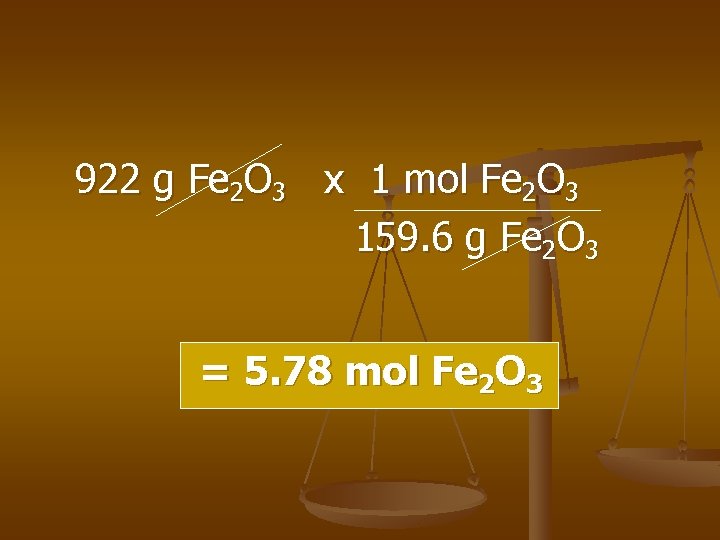








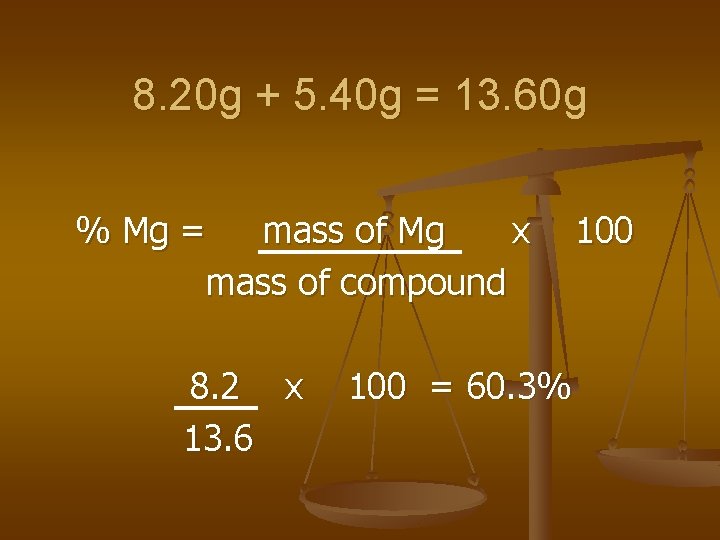













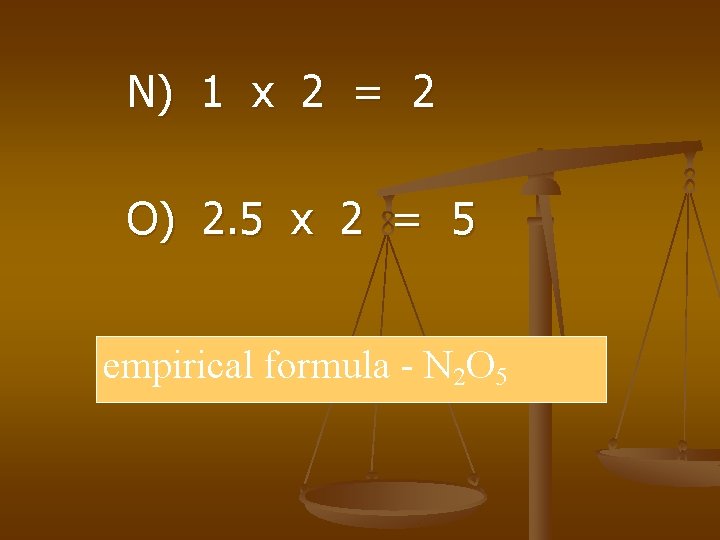





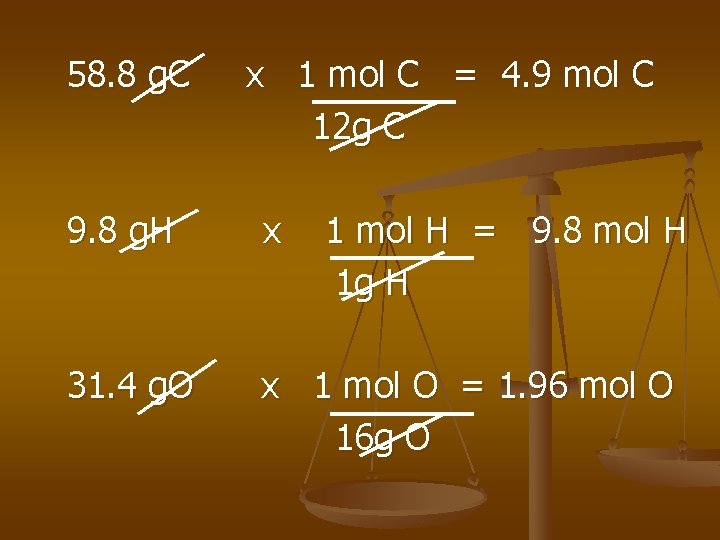
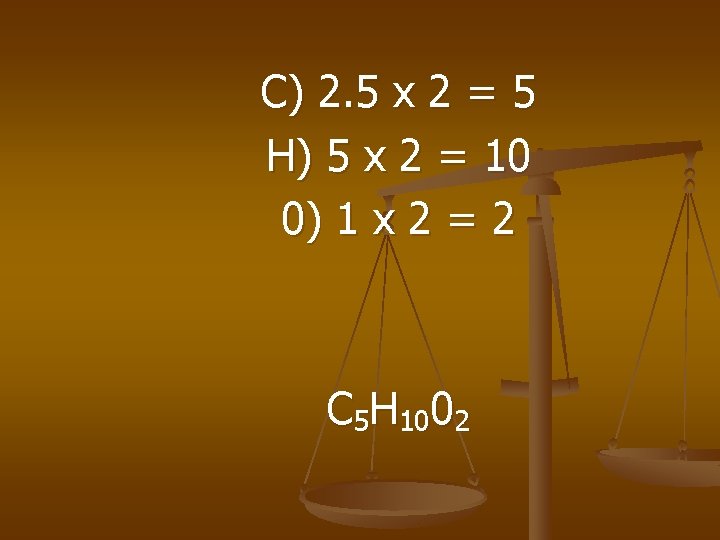
- Slides: 70

Chapter 7

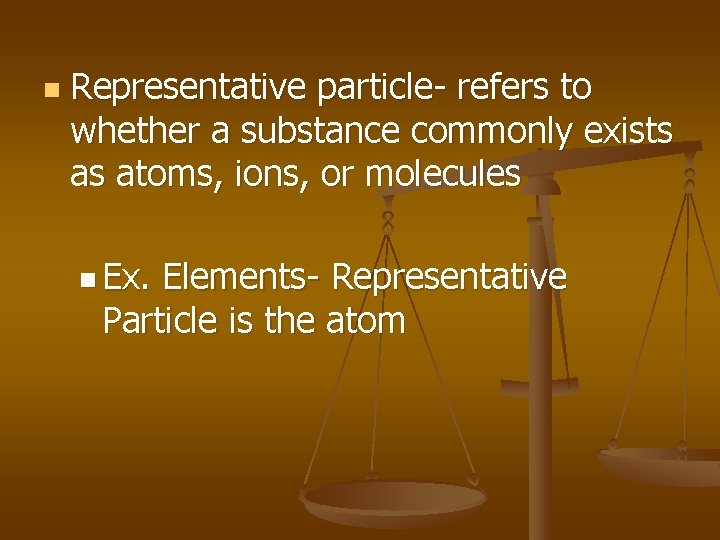
n Representative particle- refers to whether a substance commonly exists as atoms, ions, or molecules n Ex. Elements- Representative Particle is the atom

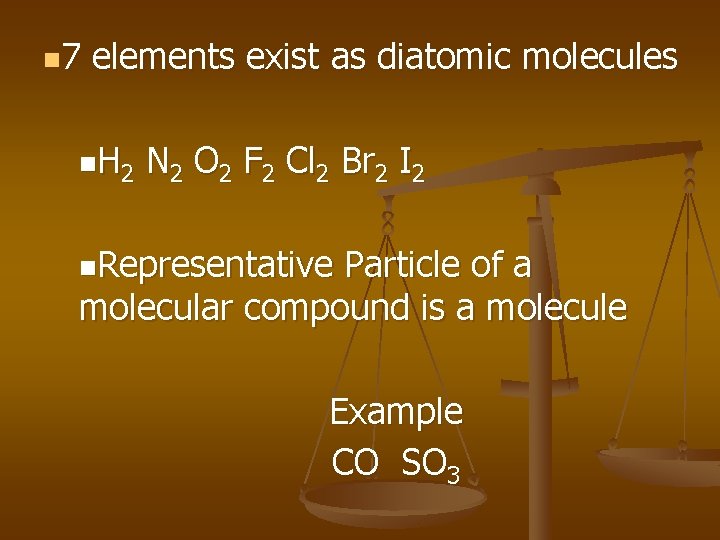
n 7 elements exist as diatomic molecules n H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2 n. Representative Particle of a molecular compound is a molecule Example CO SO 3

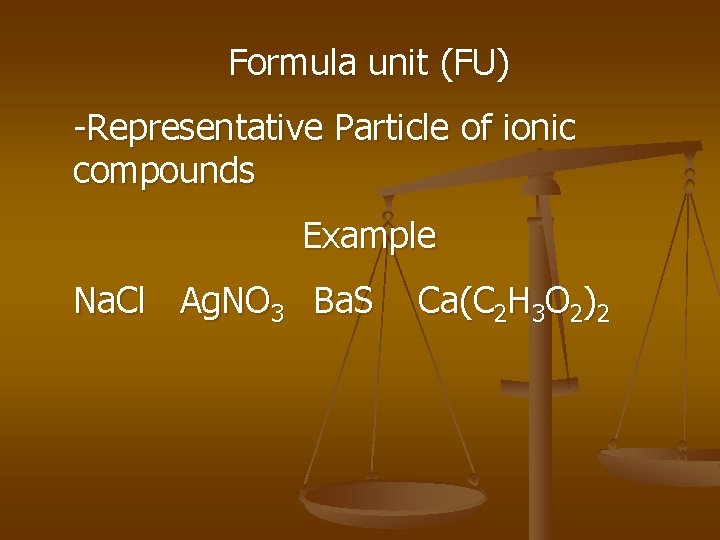
Formula unit (FU) -Representative Particle of ionic compounds Example Na. Cl Ag. NO 3 Ba. S Ca(C 2 H 3 O 2)2
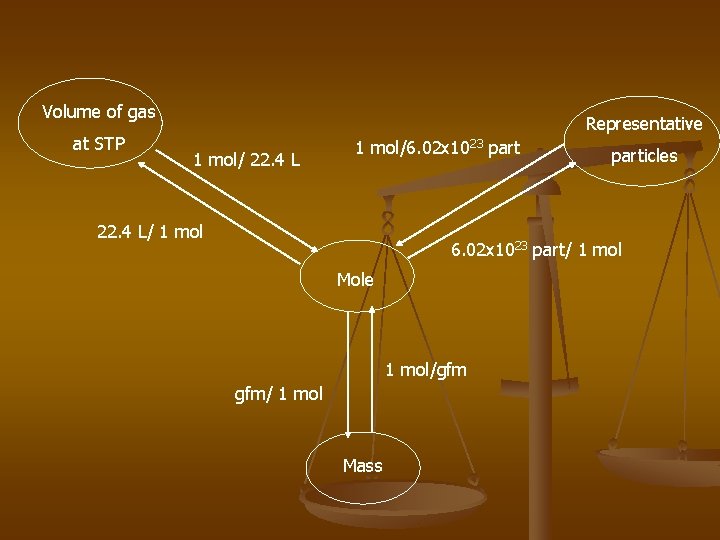
n Mole- Chemists quantity of a substance that represents 6. 02 x 1023 representative particles of that substance- called Avagadro’s number n ex. 1 dozen eggs n How many moles of Mg atoms in 3. 01 x 1022 atoms of Mg? n# of moles in 1. 20 x 1025 atoms of P?
n# of atoms in. 750 mol of Zn? n# of molecules in 4 mol of glucose, C 6 H 12 O 6? n# of moles in 1. 20 x 1024 molecules of CO 2 ?

n To find the # of atoms in 1 mole of a compound, you must determine the # of atoms in a representative formula of that compound

n# of fluoride ions in 1. 46 mol of Aluminum fluoride? n# of C atoms in a mixture of 3 mol C 2 H 2 and. 7 mol carbon monoxide?

n Atomic Mass – amu n Mass of single atom n Ex) C = 12 amu n Ex) H = 1 amu (Periodic Table)

n Gram Atomic Mass – gam n # of grams of an element that is numerically equal to the atomic mass in amu. n Ex) Carbon – gam is 12 g n Ex) Oxygen – gam is 16 g

n GAM – mass of 1 mol of atoms of a mono-atomic element n Ex) Carbon – gam is 12 g/mol n Ex) Oxygen – gam is 16 g/mol

n GMM – mass of 1 mol of that compound. n Ex) GMM of H 2 O 2
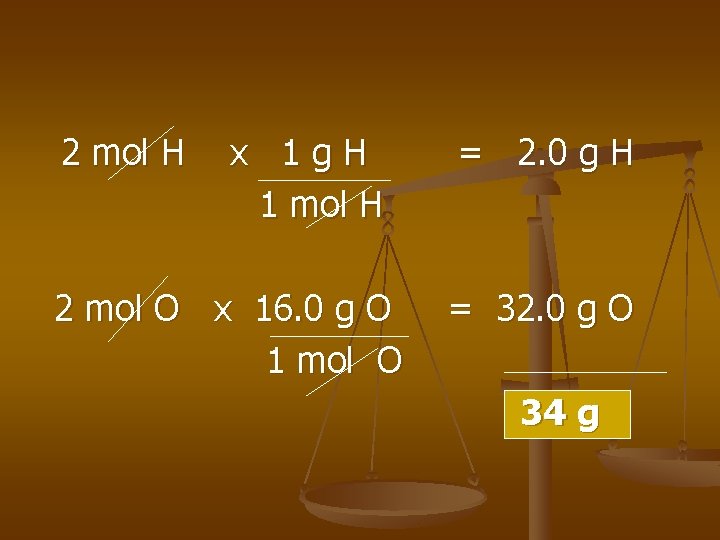
2 mol H x 1 g. H 1 mol H 2 mol O x 16. 0 g O 1 mol O = 2. 0 g H = 32. 0 g O 34 g

Examples n Find the GMM of C 6 H 4 Cl 2
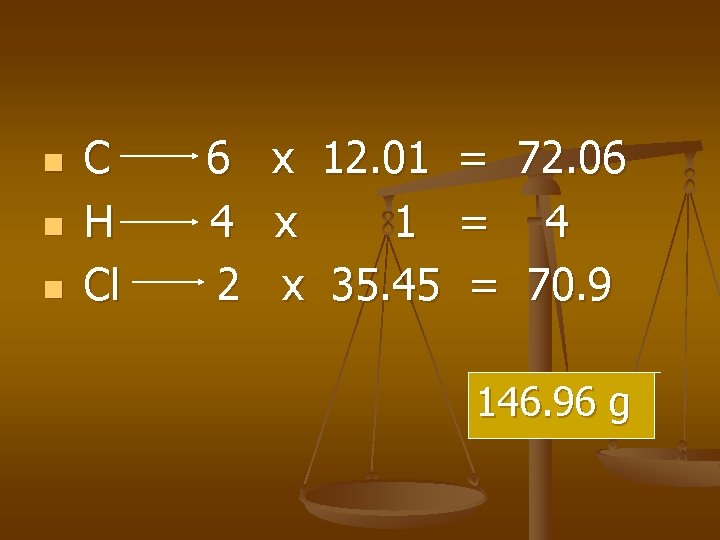
n n n C H Cl 6 x 12. 01 = 72. 06 4 x 1 = 4 2 x 35. 45 = 70. 9 146. 96 g

n GFM – Mass of 1 mol of an ionic compound. n Ex) GFM of Ammonium Carbonate

2 mol N x 8 mol H x 1 mol C x 3 mol O x 14 g. N 1 mol N 1 g. H 1 mol H 12 g C 1 mol C 16 g O 1 mol O = 28 g = 8 g = 12 g = 48 g 96 g
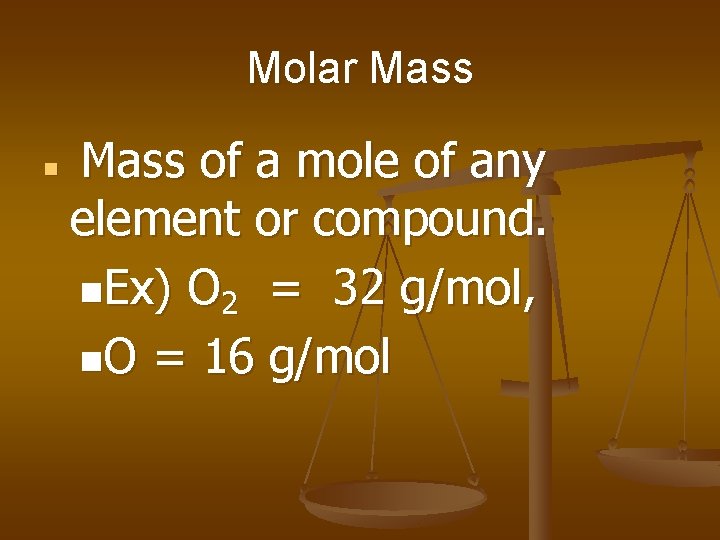
Molar Mass n Mass of a mole of any element or compound. n. Ex) O 2 = 32 g/mol, n. O = 16 g/mol

Mole Mass Conversion Ex) # of grams in 7. 20 mole of dinitrogen trioxide n
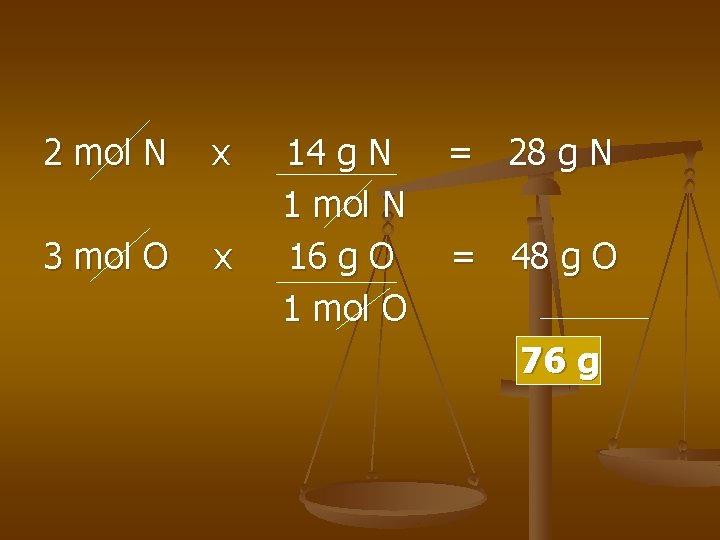
2 mol N x 3 mol O x 14 g N 1 mol N 16 g O 1 mol O = 28 g N = 48 g O 76 g

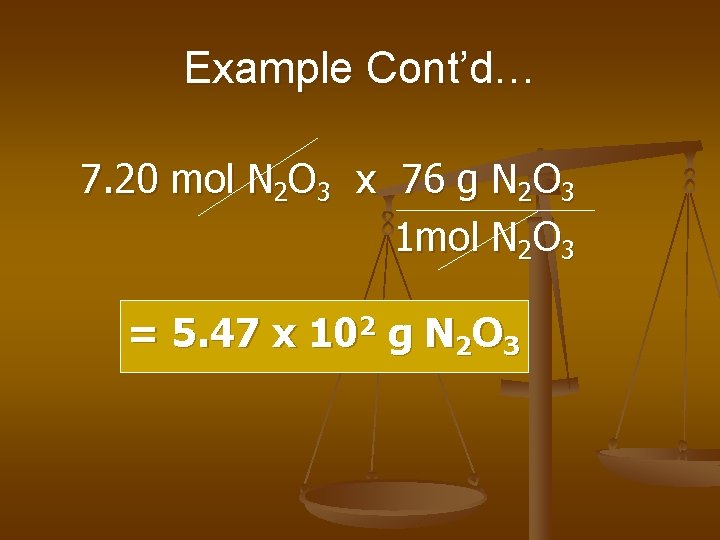
Example Cont’d… 7. 20 mol N 2 O 3 x 76 g N 2 O 3 1 mol N 2 O 3 = 5. 47 x 102 g N 2 O 3

Grams n Moles Ex) find # of moles 922 g of iron(III) oxide.
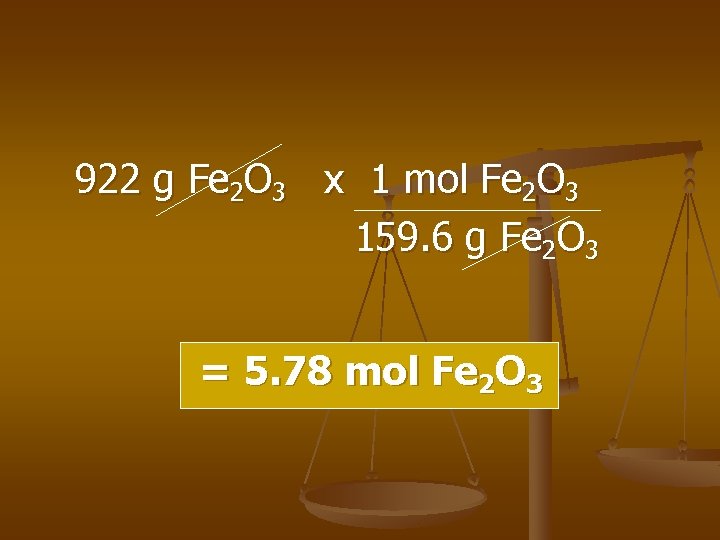
922 g Fe 2 O 3 x 1 mol Fe 2 O 3 159. 6 g Fe 2 O 3 = 5. 78 mol Fe 2 O 3

n The Volume of a gas at Standard Temperature and Pressure is 22. 4 L (STP). n Std Temp = 0°C n Std Press = 1 atmosphere (atm) n 22. 4 = molar volume of a gas = 22. 4 L 1 mol

n Ex) Determine the Volume in L of 0. 600 mol of Sulfur Dioxide gas @ STP. n Mol L n Known : 1 mol SO 2 = 22. 4 L

. 600 mol SO 2 x 22. 4 L 1 mol SO 2 = 13. 4 L SO 2

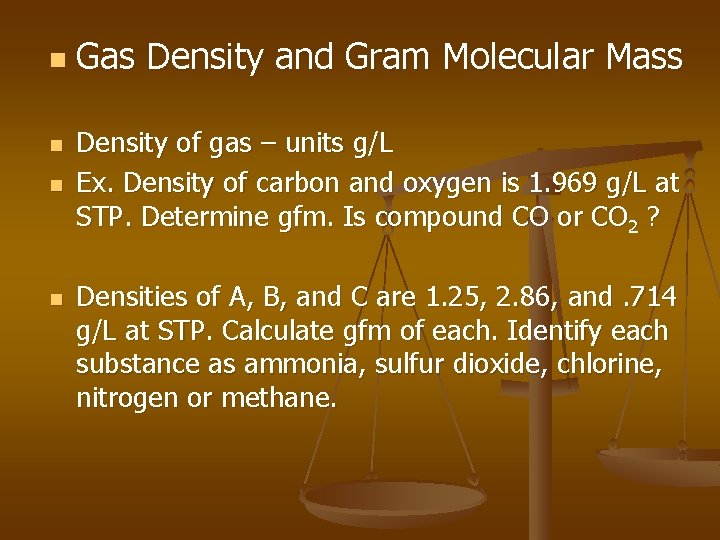
n n Gas Density and Gram Molecular Mass Density of gas – units g/L Ex. Density of carbon and oxygen is 1. 969 g/L at STP. Determine gfm. Is compound CO or CO 2 ? Densities of A, B, and C are 1. 25, 2. 86, and. 714 g/L at STP. Calculate gfm of each. Identify each substance as ammonia, sulfur dioxide, chlorine, nitrogen or methane.

Volume of gas at STP Representative 1 mol/ 22. 4 L 1 mol/6. 02 x 1023 part 22. 4 L/ 1 mol particles 6. 02 x 1023 part/ 1 mol Mole 1 mol/gfm gfm/ 1 mol Mass

n Ex. How many Carbon atoms are in a 50 carat diamond that is pure carbon? n n n 50 carats= 10 g Mass in grams of an atom of nickel? How many molecules are in a 6 L balloon filled with carbon dioxide (@ STP)?

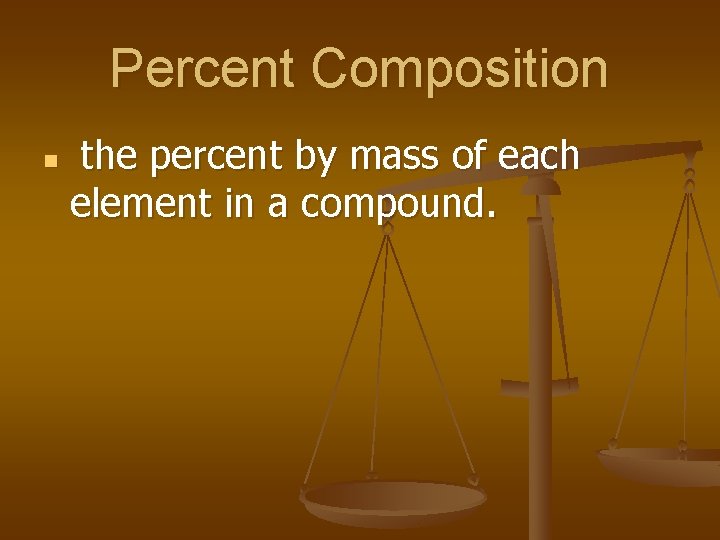
Percent Composition n the percent by mass of each element in a compound.

Examples n Find the percent composition of K 2 Cr. O 4

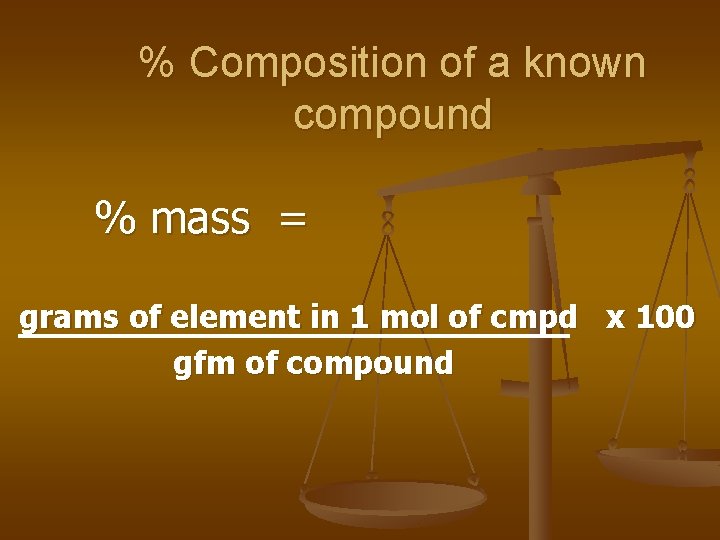
% mass = grams of element x 100 grams of compound

n 40. 3 %K n 26. 8 % Cr n 32. 9 % O n They must add up to equal 100%

Example n An 8. 20 g piece of Mg combines completely with 5. 40 g of oxygen to form a compound. Calculate the % composition of the compound.
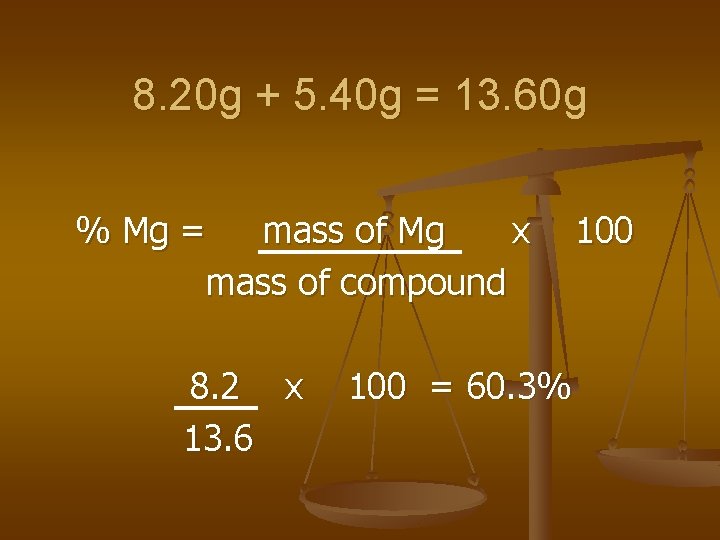
8. 20 g + 5. 40 g = 13. 60 g % Mg = mass of Mg x mass of compound 8. 2 x 13. 6 100 = 60. 3% 100

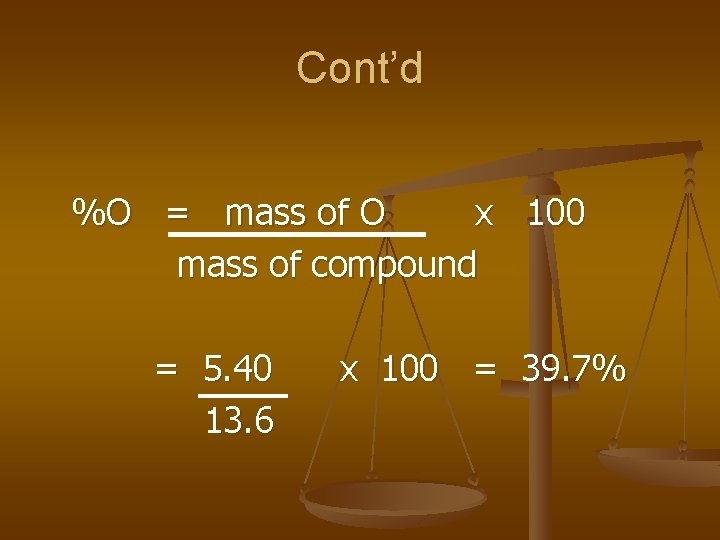
Cont’d %O = mass of O x 100 mass of compound = 5. 40 13. 6 x 100 = 39. 7%

Example n 29 g of silver combines with 4. 3 g of sulfur. Calculate % composition.

29 x 33. 3 4. 3 x 33. 3 100 = 87. 1% Ag = 12. 9%S

Example n 222. 6 g of Sodium combines with 77. 4 g of Oxygen. Calculate % composition.

222. 6 300 x 100 = 74. 2 % Na 77. 4 300 x 100 = 25. 8% O

% Composition of a known compound % mass = grams of element in 1 mol of cmpd x 100 gfm of compound

Examples n Calculate the % composition of ethane, C 2 H 6.

Cont’d n. C n. H - 2 x 12 = 24 g 6 x 1 = 6 g 30 g

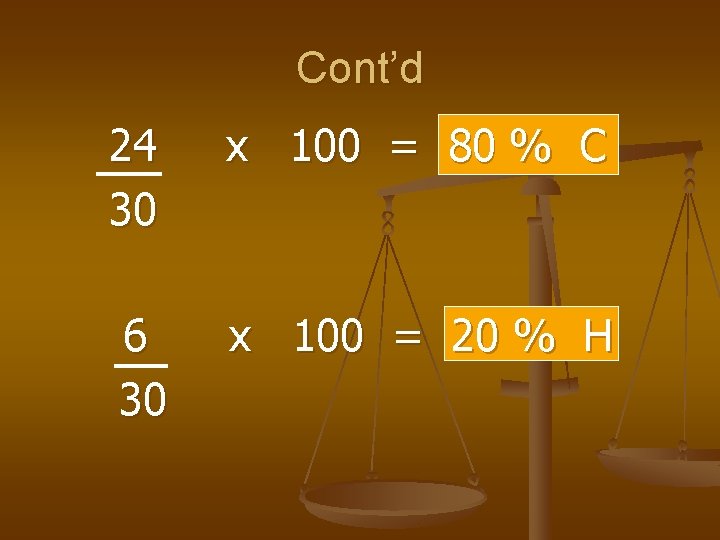
Cont’d 24 30 x 100 = 80 % C 6 30 x 100 = 20 % H

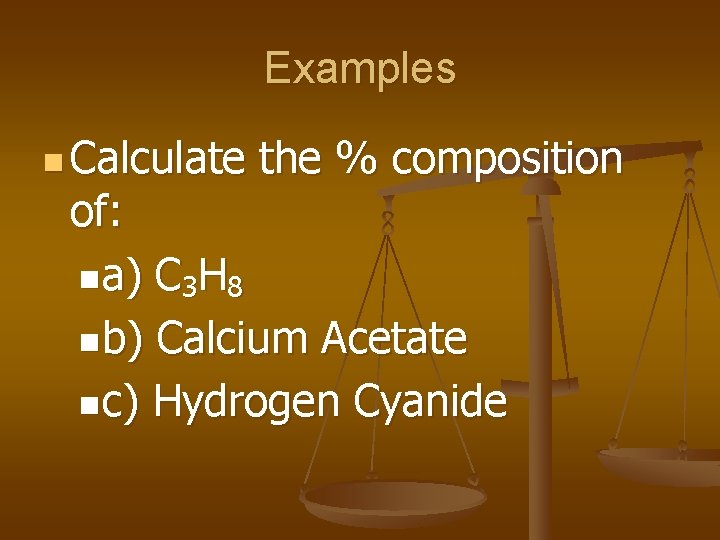
Examples n Calculate the % composition of: na) C 3 H 8 nb) Calcium Acetate nc) Hydrogen Cyanide

n a) 81. 8%C, 18. 2% H n b) 25. 4% Ca, 30. 4% C, 3. 8% H, 40. 5% O n c) 3. 7% H, 44. 4% C, 51. 9% N

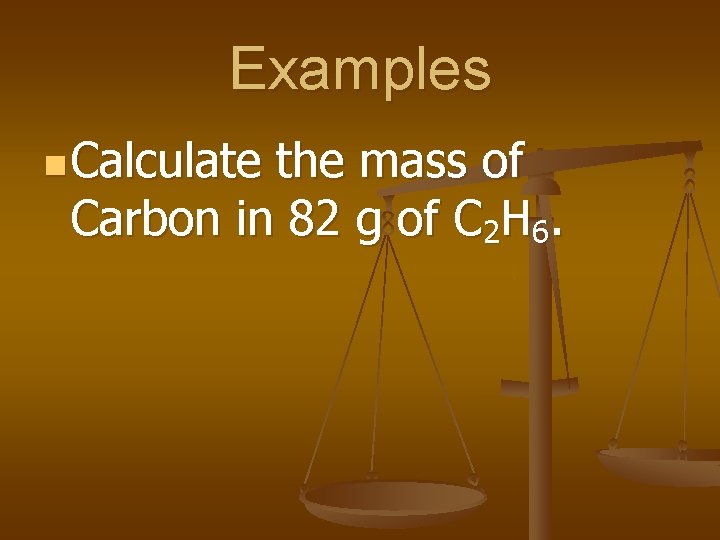
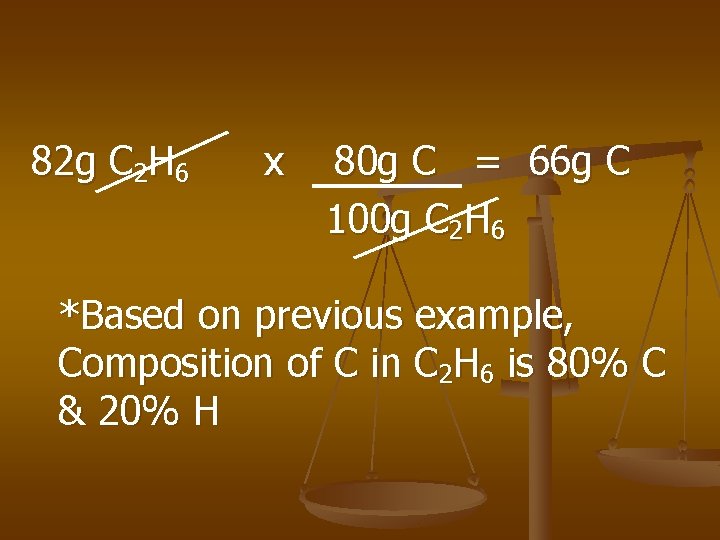
Examples n Calculate the mass of Carbon in 82 g of C 2 H 6.

82 g C 2 H 6 x 80 g C = 66 g C 100 g C 2 H 6 *Based on previous example, Composition of C in C 2 H 6 is 80% C & 20% H

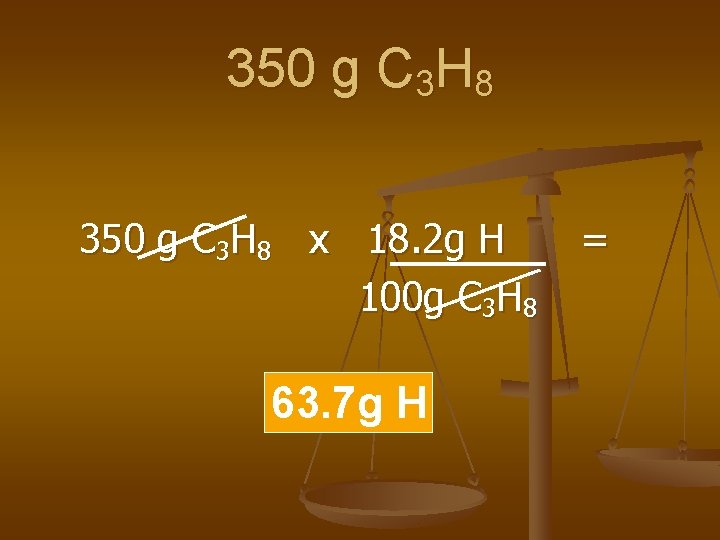
Examples n Calculate the amount of Hydrogen in the following compounds.

350 g C 3 H 8 x 18. 2 g H 100 g C 3 H 8 63. 7 g H =

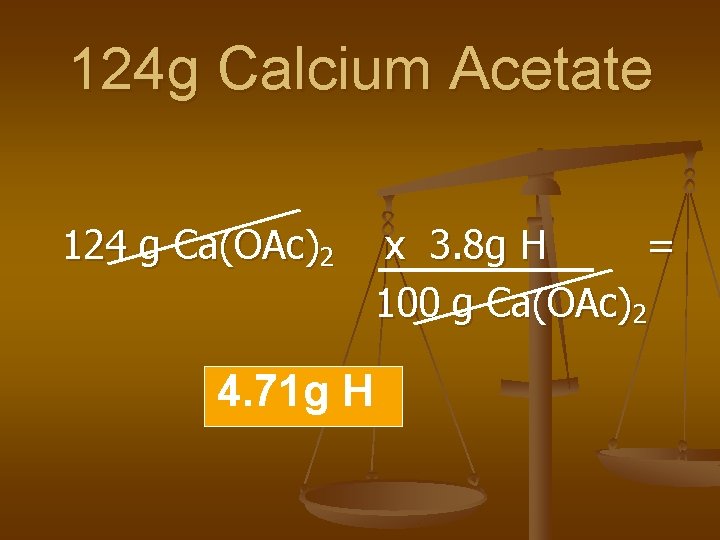
124 g Calcium Acetate 124 g Ca(OAc)2 4. 71 g H x 3. 8 g H = 100 g Ca(OAc)2

378 g Hydrogen Cyanide 378 g HCN x 3. 7 g H = 100 g HCN 14 g H

Empirical Formulas

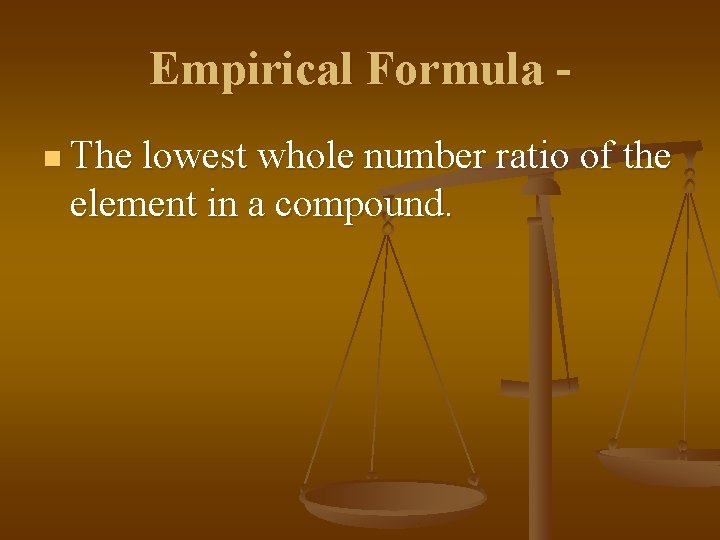
Empirical Formula n The lowest whole number ratio of the element in a compound.

n An empirical formula may not be the same as the molecular formula n Examples n CO 2 is both empirical and molecular n N 2 H 4 is molecular formula n NH 2 is empirical formula because it is the simplest ratio of N : H

Examples n Find the empirical formula of a compound that is 25. 9% nitrogen and 74. 1% oxygen.

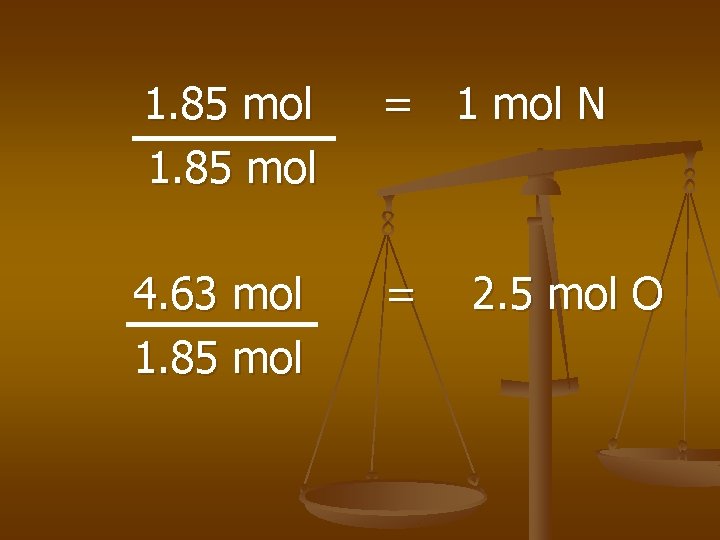
25. 9 g N x 1 mol N 14 g N = 1. 85 mol N 74. 1 g O x = 4. 63 mol O 16 g O

1. 85 mol = 1 mol N 4. 63 mol 1. 85 mol = 2. 5 mol O
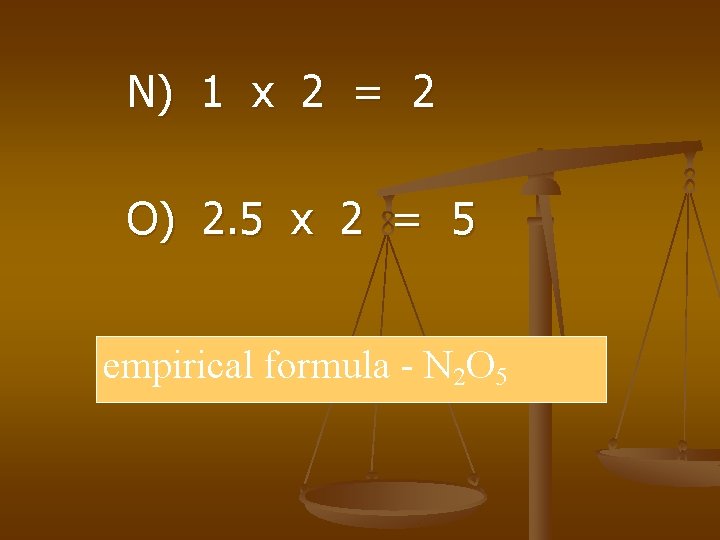
N) 1 x 2 = 2 O) 2. 5 x 2 = 5 empirical formula - N 2 O 5

Examples n Find the empirical formulas for the following compounds.

79. 8 %C , 20. 2 %H n. Empirical CH 3 Formula

67. 6% Mercury , 10. 8% Sulfur, 21. 6% Oxygen n Empirical Formula Hg. SO 4

17. 6% Sodium, 39. 7% Chromium, 42. 7% Oxygen n Empirical Formula Na 2 Cr 2 O 7

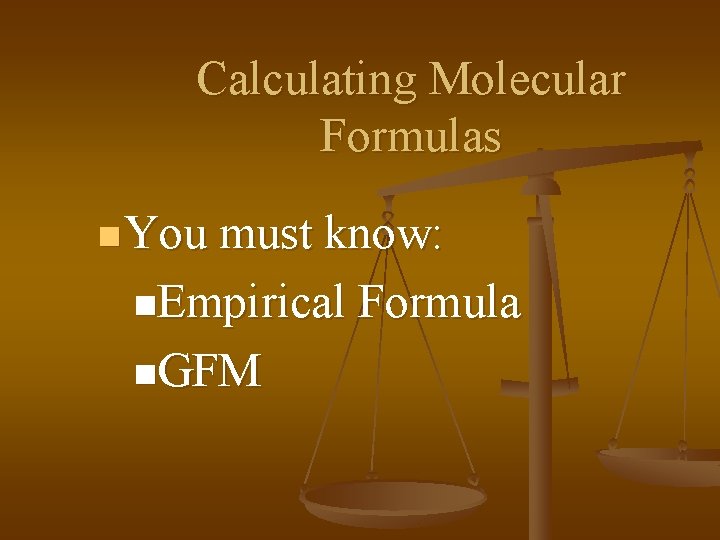
Calculating Molecular Formulas n You must know: n. Empirical Formula n. GFM

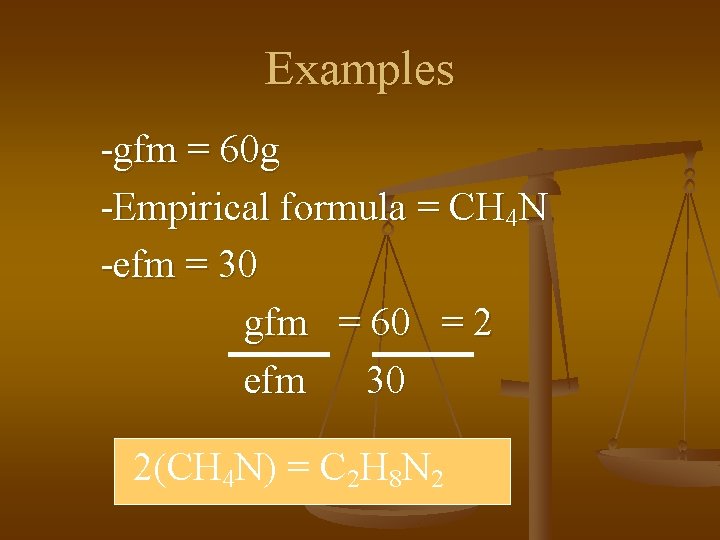
Examples -gfm = 60 g -Empirical formula = CH 4 N -efm = 30 gfm = 60 = 2 efm 30 2(CH 4 N) = C 2 H 8 N 2

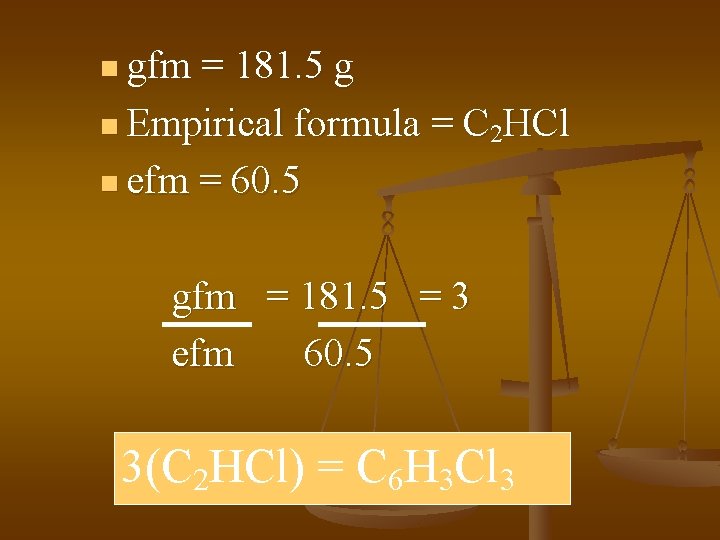
n gfm = 181. 5 g n Empirical formula = C 2 HCl n efm = 60. 5 gfm = 181. 5 = 3 efm 60. 5 3(C 2 HCl) = C 6 H 3 Cl 3

Methyl butanoate smells like apples. The Percent Composition is 58. 8% C, 9. 8%H, 31. 4% O. The gfm is 102 g/mol - what is the molecular formula?
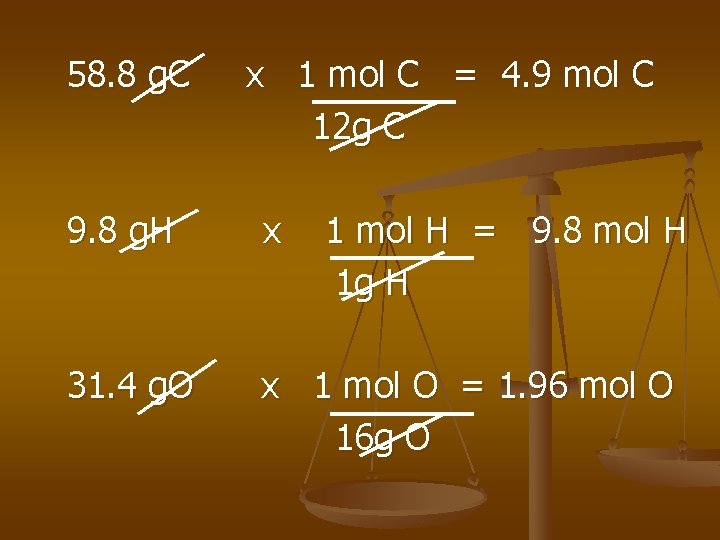
58. 8 g. C x 1 mol C = 4. 9 mol C 12 g C 9. 8 g. H x 1 mol H = 9. 8 mol H 1 g H 31. 4 g. O x 1 mol O = 1. 96 mol O 16 g O

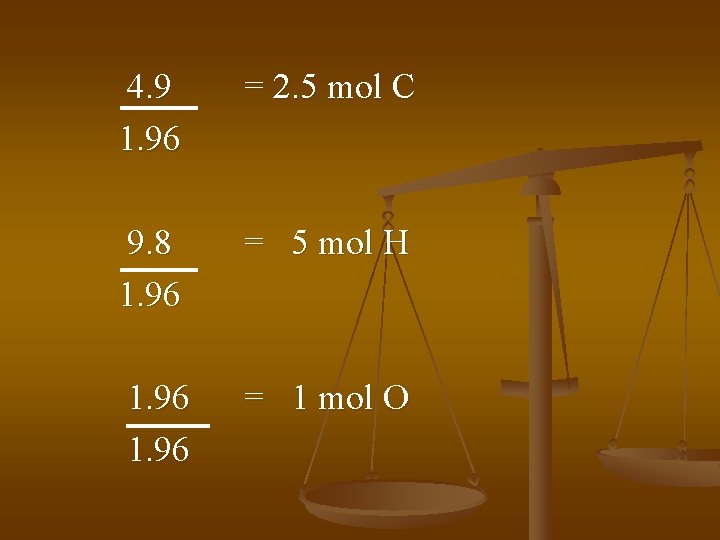
4. 9 1. 96 = 2. 5 mol C 9. 8 1. 96 = 5 mol H 1. 96 = 1 mol O
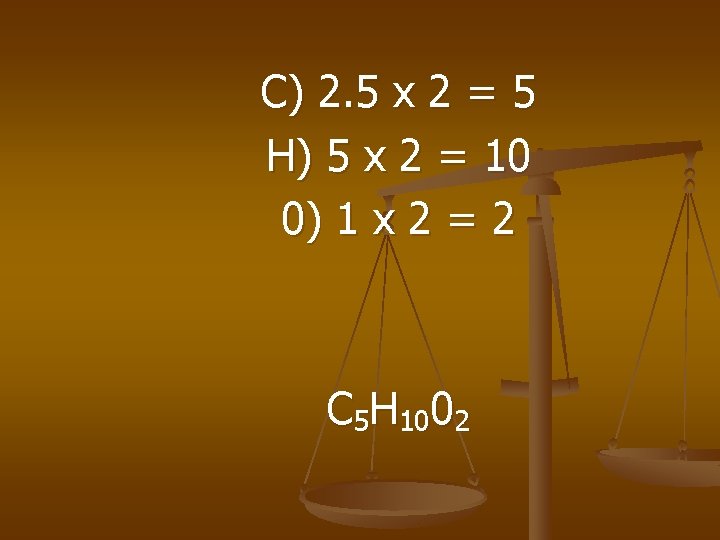
C) 2. 5 x 2 = 5 H) 5 x 2 = 10 0) 1 x 2 = 2 C 5 H 1002