Subatomic particles Name Symbol Relative Charge mass Actual
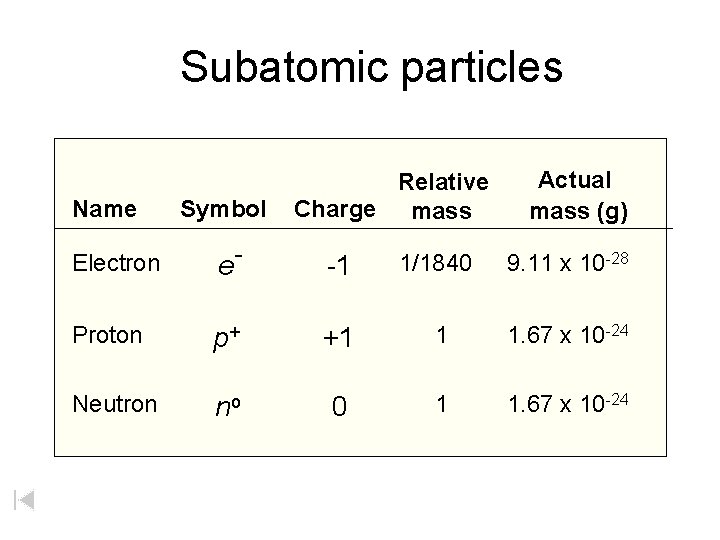
Subatomic particles Name Symbol Relative Charge mass Actual mass (g) Electron e- -1 1/1840 9. 11 x 10 -28 Proton p+ +1 1 1. 67 x 10 -24 Neutron no 0 1 1. 67 x 10 -24

The Atomic Scale § Most of the mass of the atom is in the nucleus (protons and neutrons) 99% § Electrons are found outside of the nucleus (the electron cloud) § Most of the volume of the atom is empty space “q” is a particle called a “quark”


A. Mass Number • weighted average of all isotopes • rounded to 2 decimal places • mass # = protons + neutrons • always a whole number • NOT a whole number on the Periodic Table! © Addison-Wesley Publishing Company, Inc.

Atomic Number Atomic number of an element is the number of protons in the nucleus of each atom of that element. Also equals the number of electrons. Element Carbon Phosphorus Gold # of electrons 6 15 # of protons 6 15 Atomic # 79 79 79 6 15

B. Isotopes • Atoms of the same element with different mass numbers. Same number of protons, different number of neutrons. • Nuclear symbol: Mass # Atomic # • Hyphen notation: carbon-12
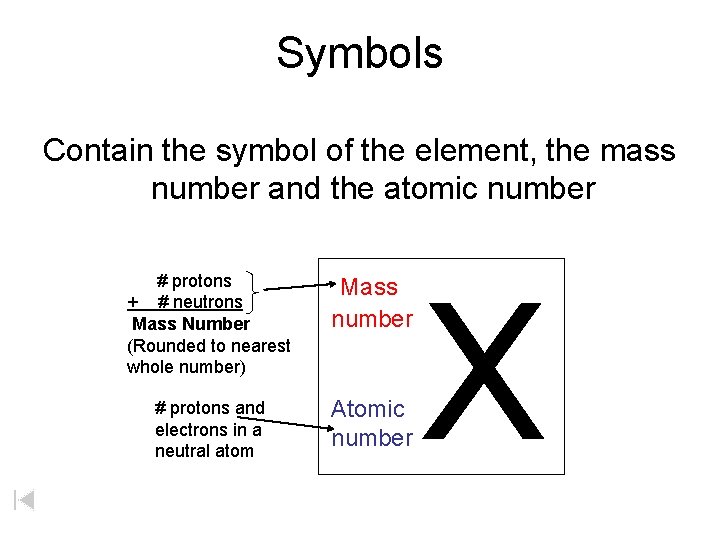
Symbols Contain the symbol of the element, the mass number and the atomic number # protons + # neutrons Mass Number (Rounded to nearest whole number) Mass number # protons and electrons in a neutral atom Atomic number X

Symbols • Find the – number of protons = 9 + – number of neutrons = 10 – number of electrons = 9 – Atomic number = 9 – Mass number = 19 19 9 F
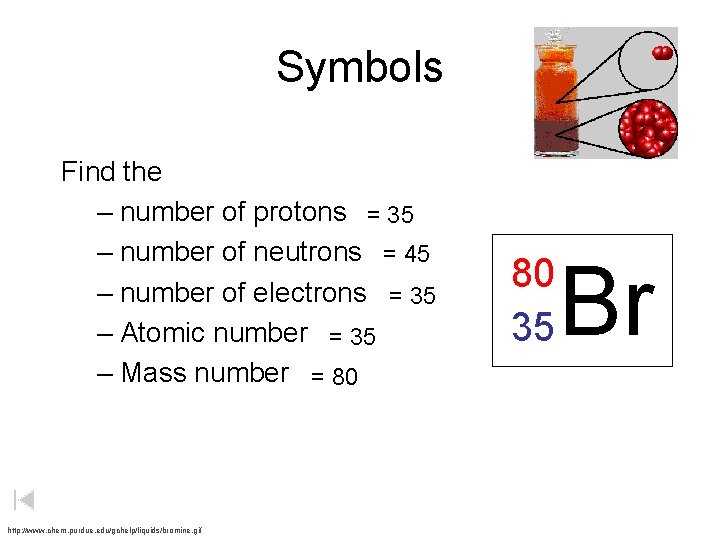
Symbols Find the – number of protons = 35 – number of neutrons = 45 – number of electrons = 35 – Atomic number = 35 – Mass number = 80 http: //www. chem. purdue. edu/gchelp/liquids/bromine. gif 80 35 Br

Symbols Find the – number of protons = 11 – number of neutrons = 12 – number of electrons = 11 – Atomic number = 11 – Mass number = 23 23 11 Na Sodium atom
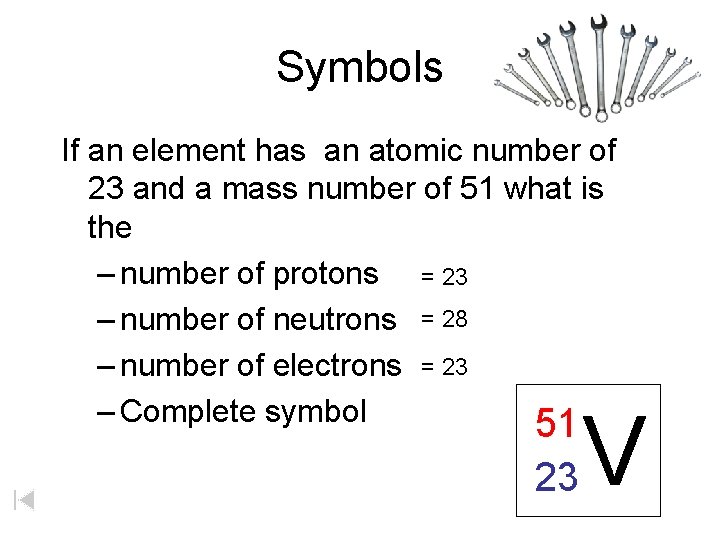
Symbols If an element has an atomic number of 23 and a mass number of 51 what is the – number of protons = 23 – number of neutrons = 28 – number of electrons = 23 – Complete symbol 51 23 V

Symbols If an element has 60 protons and 84 neutrons what is the – Atomic number = 60 = 144 – Mass number – number of electrons = 60 – Complete symbol Nd 144 60

Symbols If a neutral atom of an element has 78 electrons and 117 neutrons what is the – Atomic number = 78 – Mass number = 195 – number of protons = 78 – Complete symbol Pt 195 78
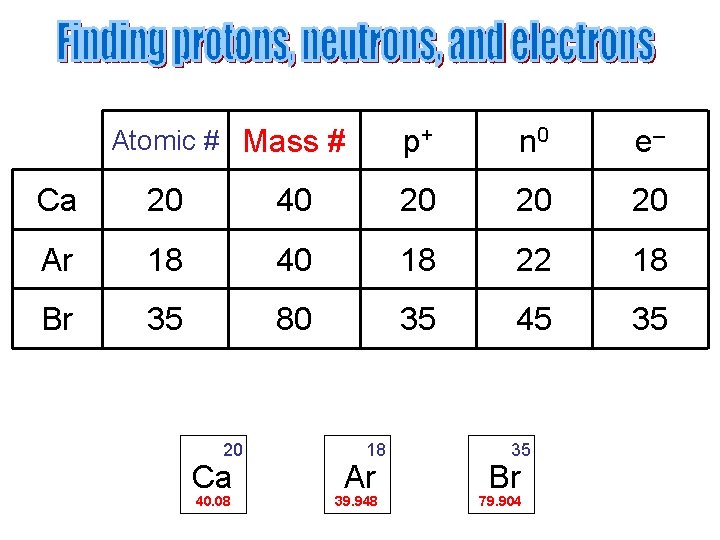
Atomic # Mass # p+ n 0 e– Ca 20 40 20 20 20 Ar 18 40 18 22 18 Br 35 80 35 45 35 20 Ca 40. 08 18 Ar 39. 948 35 Br 79. 904
- Slides: 14