How many protons electrons and neutrons are in

- Slides: 10

How many protons, electrons, and neutrons are in an atom? http: //physicsed. buffalostate. edu/Wiley/CJ 6 e/img 30/PTE. gif

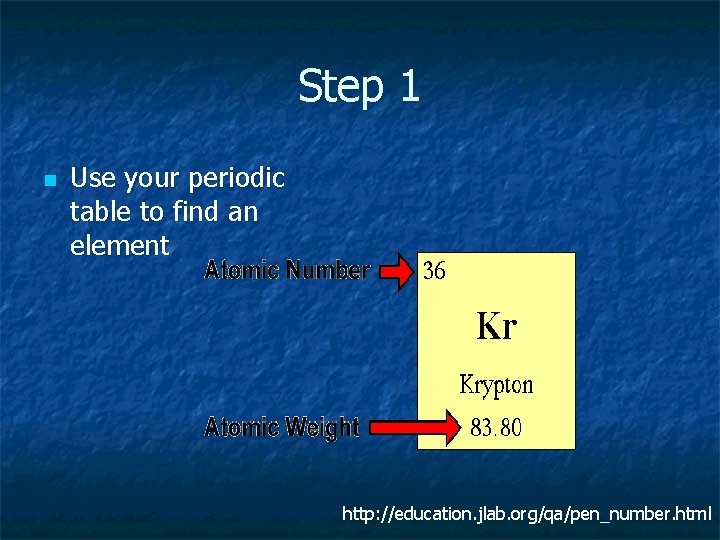
Step 1 n Use your periodic table to find an element http: //education. jlab. org/qa/pen_number. html

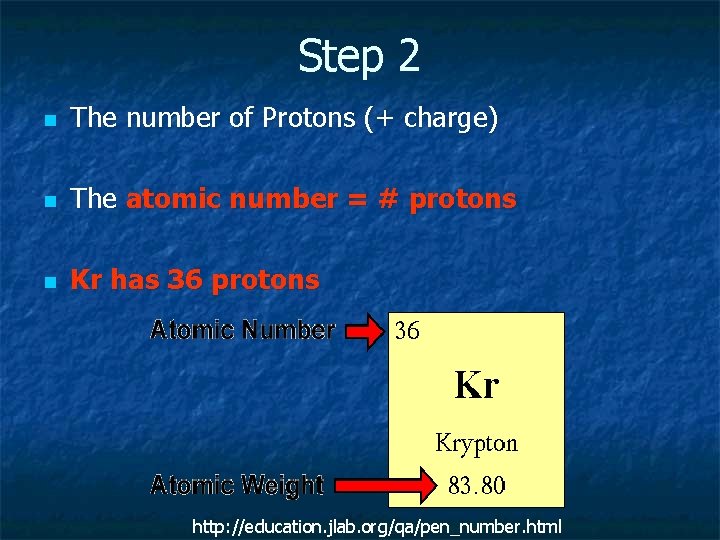
Step 2 n The number of Protons (+ charge) n The atomic number = # protons n Kr has 36 protons http: //education. jlab. org/qa/pen_number. html

Step 3 n n n The number of Electrons (- charge) Remember atoms have no overall charge. Atoms must have an equal # of protons and electrons #protons=#electrons Kr has 36 electrons

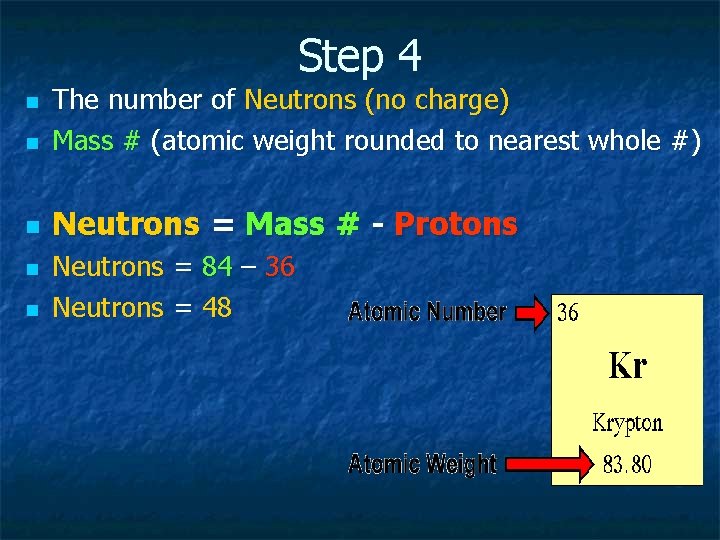
Step 4 n The number of Neutrons (no charge) Mass # (atomic weight rounded to nearest whole #) n Neutrons = Mass # - Protons n n n Neutrons = 84 – 36 Neutrons = 48

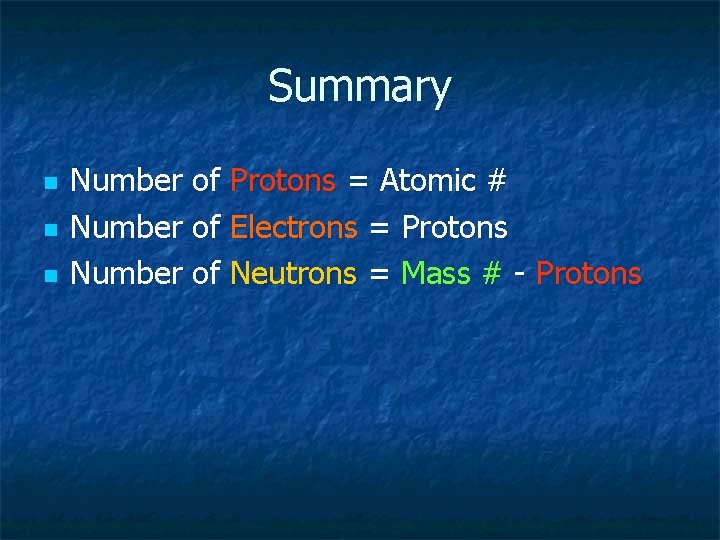
Summary n n n Number of Protons = Atomic # Number of Electrons = Protons Number of Neutrons = Mass # - Protons

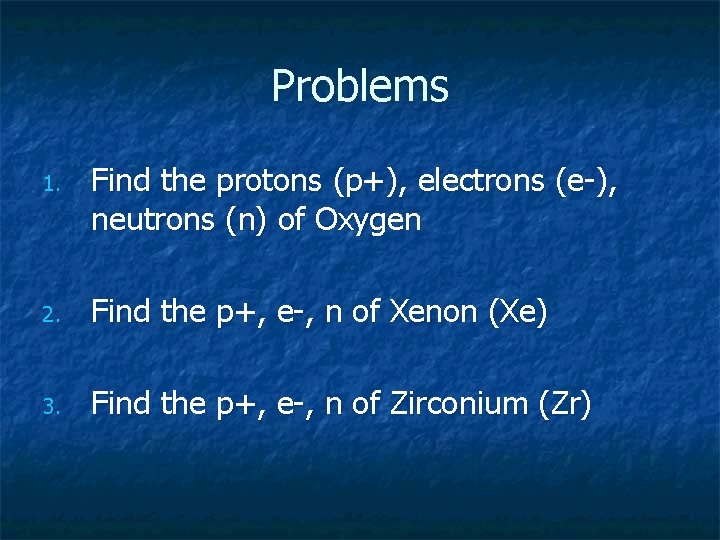
Problems 1. Find the protons (p+), electrons (e-), neutrons (n) of Oxygen 2. Find the p+, e-, n of Xenon (Xe) 3. Find the p+, e-, n of Zirconium (Zr)

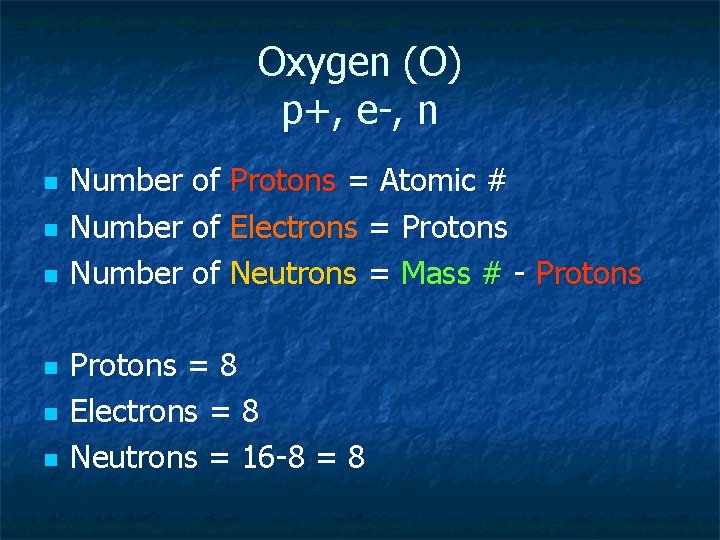
Oxygen (O) p+, e-, n n n n Number of Protons = Atomic # Number of Electrons = Protons Number of Neutrons = Mass # - Protons = 8 Electrons = 8 Neutrons = 16 -8 = 8

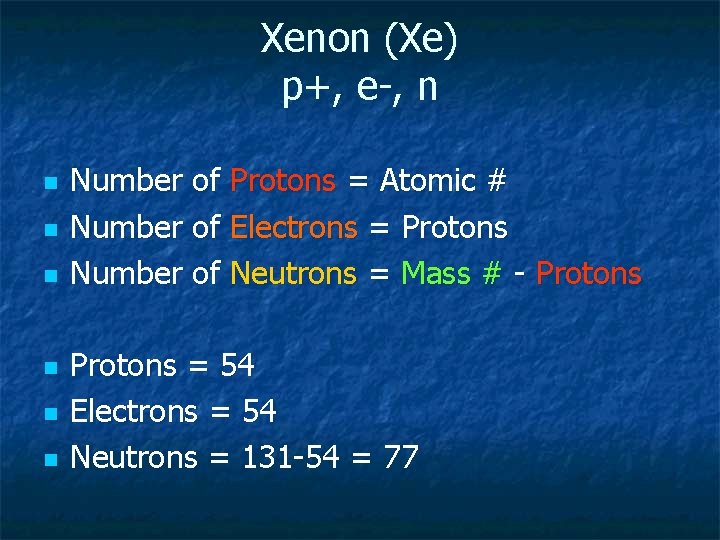
Xenon (Xe) p+, e-, n n n n Number of Protons = Atomic # Number of Electrons = Protons Number of Neutrons = Mass # - Protons = 54 Electrons = 54 Neutrons = 131 -54 = 77

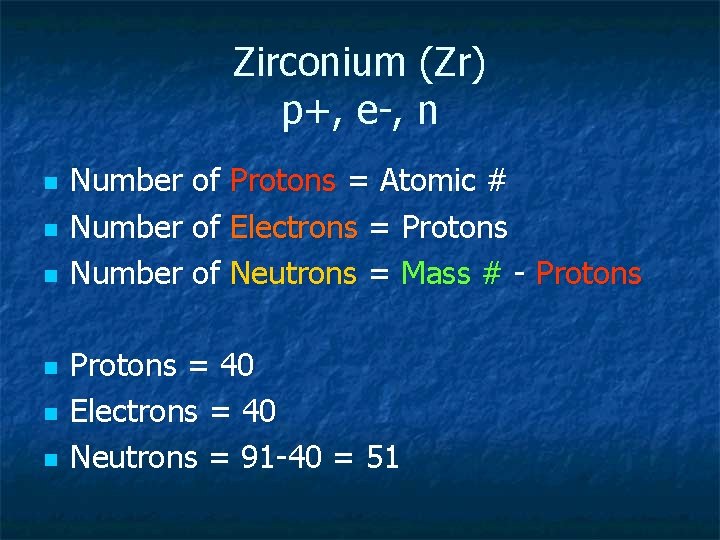
Zirconium (Zr) p+, e-, n n n n Number of Protons = Atomic # Number of Electrons = Protons Number of Neutrons = Mass # - Protons = 40 Electrons = 40 Neutrons = 91 -40 = 51
 Sulfur number of neutrons protons and electrons
Sulfur number of neutrons protons and electrons Find the number of protons os
Find the number of protons os Ytterbium atomic number
Ytterbium atomic number Basic atomic structure worksheet
Basic atomic structure worksheet What is an atom inventory
What is an atom inventory How to find protons
How to find protons Lithium protons neutrons electrons
Lithium protons neutrons electrons Chromium 58 protons, neutrons electrons
Chromium 58 protons, neutrons electrons 15n protons neutrons electrons
15n protons neutrons electrons Democritus atomic theory
Democritus atomic theory 39k+ protons neutrons electrons
39k+ protons neutrons electrons