DETERMINE THE NUMBER OF PROTONS NEUTRONS AND ELECTRONS
DETERMINE THE NUMBER OF PROTONS, NEUTRONS AND ELECTRONS IN AN ATOM

Counting the Pieces n Atomic Number = number of protons n # of protons determines kind of atom. n the same as the number of electrons in the neutral atom.

Counting the Pieces n Mass Number = the number of protons + neutrons. n All the things with mass. n NOT on the periodic table

Isotopes n Atoms of the same element can have different numbers of neutrons. n different mass numbers. n called isotopes.

Writing Isotopes Two ways: n Hyphen notation – Name of element – mass number n Ex: – carbon- 12 – carbon -14 – uranium-235 n
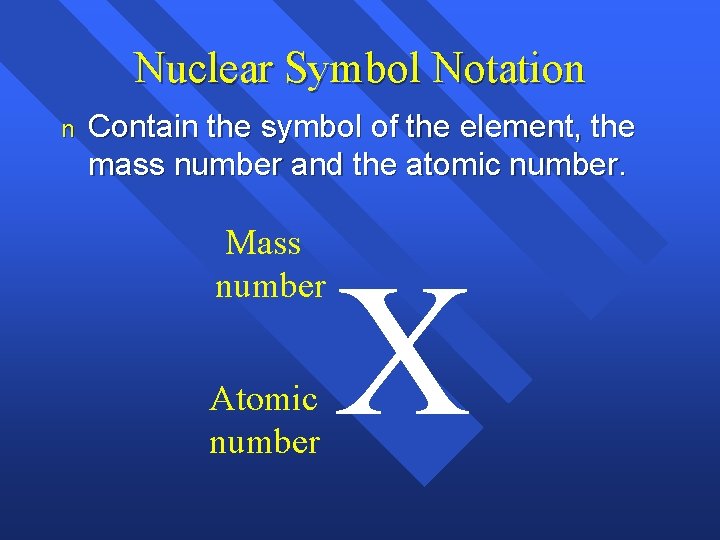
Nuclear Symbol Notation n Contain the symbol of the element, the mass number and the atomic number. Mass number Atomic number X
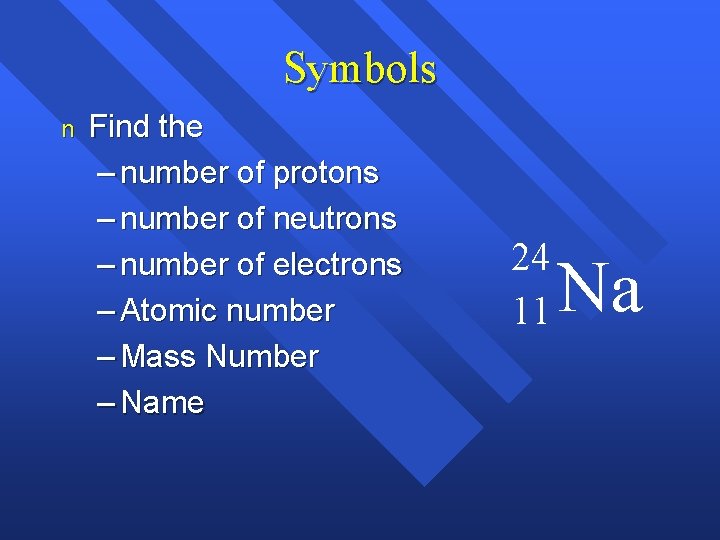
Symbols n Find the – number of protons – number of neutrons – number of electrons – Atomic number – Mass Number – Name 24 11 Na
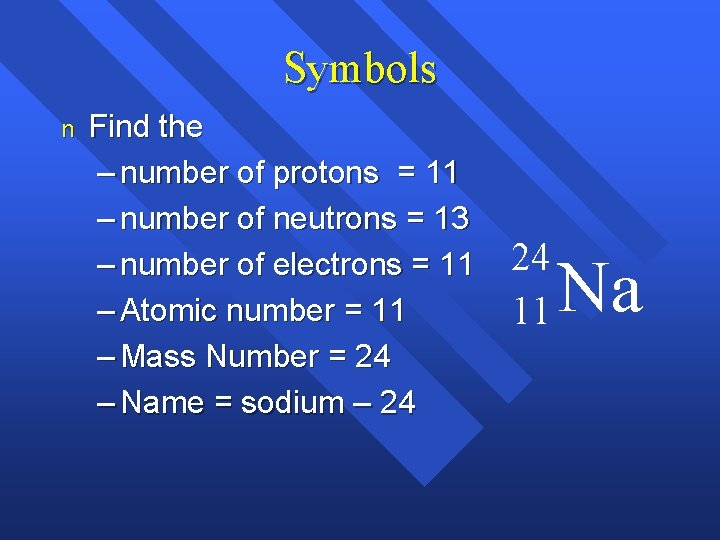
Symbols n Find the – number of protons = 11 – number of neutrons = 13 – number of electrons = 11 – Atomic number = 11 – Mass Number = 24 – Name = sodium – 24 24 11 Na
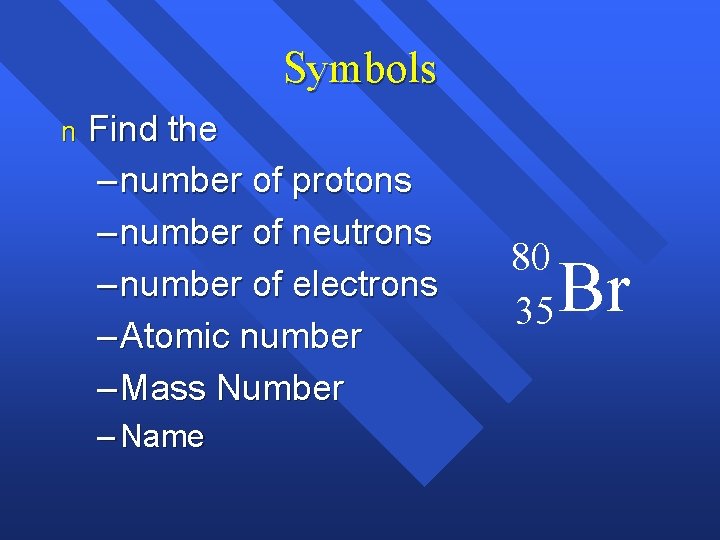
Symbols n Find the – number of protons – number of neutrons – number of electrons – Atomic number – Mass Number – Name 80 35 Br
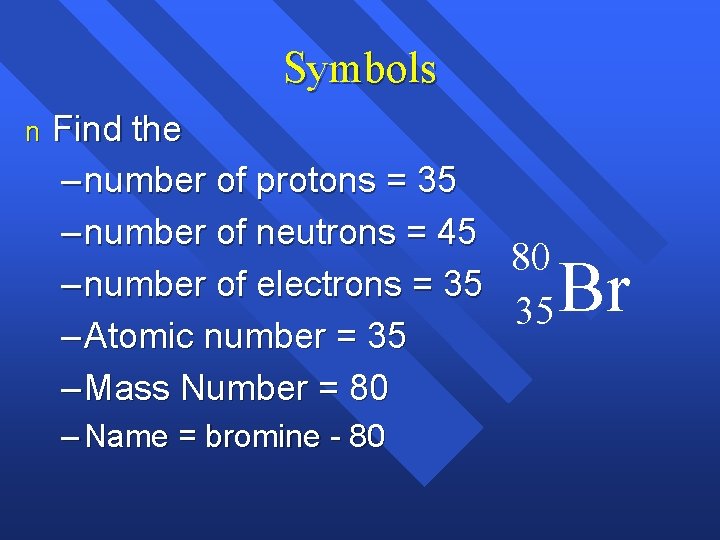
Symbols n Find the – number of protons = 35 – number of neutrons = 45 80 – number of electrons = 35 35 – Atomic number = 35 – Mass Number = 80 – Name = bromine - 80 Br
Symbols n if an element has an atomic number of 34 and a mass number of 78 what is the – number of protons – number of neutrons – number of electrons – symbol – Name

ANSWERS n if an element has an atomic number of 34 and a mass number of 78 what is the – number of protons = 34 – number of neutrons = 44 – number of electrons = 34 – Name = selenium – 78 78 34 Se
Symbols n if an element has 91 protons and 140 neutrons what is the – Atomic number – Mass number – number of electrons – Name – symbol

ANSWERS n if an element has 91 protons and 140 neutrons what is the – Atomic number = 91 – Mass number = 140 – number of electrons = 91 – Name = Protactinium - 140 91 Pa
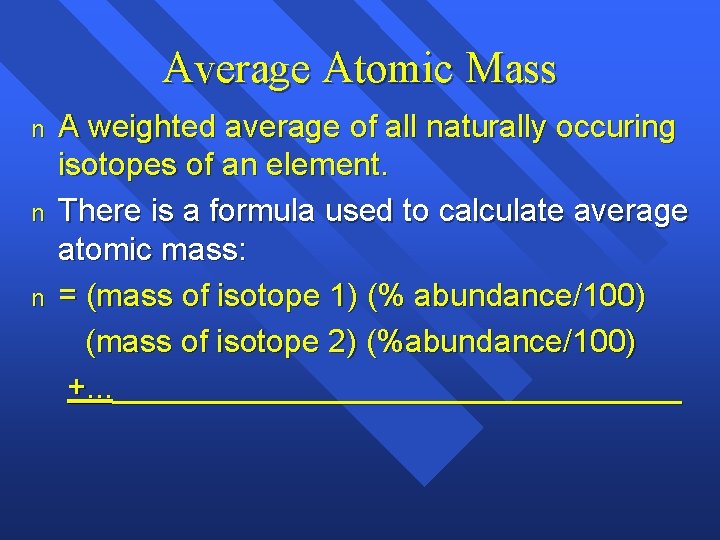
Average Atomic Mass n n n A weighted average of all naturally occuring isotopes of an element. There is a formula used to calculate average atomic mass: = (mass of isotope 1) (% abundance/100) (mass of isotope 2) (%abundance/100) +. . . ________________

Unit for Atomic Mass The atomic mass unit (amu) n amu definition - one twelfth the mass of a carbon-12 atom. n Each isotope has its own atomic mass relative to the carbon-12 atom n

Atomic Mass n You try… n Calculate the atomic mass of copper if copper has two isotopes. 69. 1% has a mass of 62. 93 amu and 30. 9% has a mass of 64. 93 amu.

Answer (62. 93 amu) (69. 1% / 100) = 43. 48 amu +(64. 93 amu) (30. 9% / 100) = 20. 06 amu 63. 54 amu

Atomic Mass n Magnesium has three isotopes. 78. 99% magnesium-24 with a mass of 23. 9850 amu, 10. 00% magnesium-25 with a mass of 24. 9858 amu, and 11. 01% magnesium-25 with a mass of 25. 9826 amu. What is the atomic mass of magnesium?

Answer (23. 9850 amu) (78. 99% / 100) = 18. 9457 (24. 9858 amu) (10. 00% / 100) = 2. 49858 (25. 9826 amu) (11. 01% / 100) = 2. 86068 24. 304 amu

Atomic Mass n n Is not a whole number because it is an average. are the decimal numbers on the periodic table.
- Slides: 21