Self Quiz Do atoms always have an equal
























- Slides: 36

Self Quiz

Do atoms always have an equal number of protons, neutrons and electrons? 1. Yes. 2. No.

Do atoms always have an equal number of protons, neutrons and electrons? 1. Yes. 2. No.

A chemical bond is formed through: 1. 2. 3. 4. 5. The gaining, losing, or sharing of protons. The gaining, losing, or sharing of neutrons. The gaining, losing, or sharing of electrons. The gaining, losing, or sharing of isotopes. The gaining, losing, or sharing of ions.

A chemical bond is formed through: 1. 2. 3. 4. 5. The gaining, losing, or sharing of protons. The gaining, losing, or sharing of neutrons. The gaining, losing, or sharing of electrons. The gaining, losing, or sharing of isotopes. The gaining, losing, or sharing of ions.

After sodium loses an electron, it is: 1. 2. 3. 4. 5. A positive ion. A negative ion. A neutral ion. An isotope. A compound.

After sodium loses an electron, it is: 1. 2. 3. 4. 5. A positive ion. A negative ion. A neutral ion. An isotope. A compound.

After chlorine gains an electron, it is: 1. 2. 3. 4. 5. A positive ion. A negative ion. A neutral ion. An isotope. A compound.

After chlorine gains an electron, it is: 1. 2. 3. 4. 5. A positive ion. A negative ion. A neutral ion. An isotope. A compound.

How many electrons does sodium lose to chlorine to form an ionic bond? 1. 2. 3. 4. 1 2 3 4 5. 6. 7. 8. 5 6 7 8

How many electrons does sodium lose to chlorine to form an ionic bond? 1. 2. 3. 4. 1 2 3 4 5. 6. 7. 8. 5 6 7 8

What is the difference between a nonpolar covalent bond a polar covalent bond? 1. 2. 3. 4. A polar covalent bond results when there is unequal sharing of electrons in a molecule, whereas electrons are shared equally in a nonpolar covalent bond. A polar covalent bond has two equal sides and a nonpolar covalent bond has two different sides. A nonpolar covalent bond is positively charged and a polar covalent bond is negatively charged. A polar covalent bond is positively charged and a nonpolar covalent bond is negatively charged.

What is the difference between a nonpolar covalent bond a polar covalent bond? 1. 2. 3. 4. A polar covalent bond results when there is unequal sharing of electrons in a molecule, whereas electrons are shared equally in a nonpolar covalent bond. A polar covalent bond has two equal sides and a nonpolar covalent bond has two different sides. A nonpolar covalent bond is positively charged and a polar covalent bond is negatively charged. A polar covalent bond is positively charged and a nonpolar covalent bond is negatively charged.

A covalent chemical bond is one in which A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged. B) protons or neutrons are shared by two atoms C) electrons of one atom are transferred to another atom. D) electrons are shared by two atoms

A covalent chemical bond is one in which A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged. B) protons or neutrons are shared by two atoms C) electrons of one atom are transferred to another atom. D) electrons are shared by two atoms

A covalent bond is likely to be polar when A) the electron being shared between two atoms is much more attracted to one of the atoms than the other atom. B) the two atoms sharing electrons are different elements. C) the two atoms sharing electrons are of the same element. D) the two atoms are sharing electrons equally

A covalent bond is likely to be polar when A) the electron being shared between two atoms is much more attracted to one of the atoms than the other atom. B) the two atoms sharing electrons are different elements. C) the two atoms sharing electrons are of the same element. D) the two atoms are sharing electrons equally

The ionic bond of sodium chloride is formed when A) sodium and chlorine both lose electrons. B) chlorine gains a proton from sodium. C) sodium gains an electron from chlorine. D) sodium and chlorine share an electron pair. E) chlorine gains an electron from sodium.

The ionic bond of sodium chloride is formed when A) sodium and chlorine both lose electrons. B) chlorine gains a proton from sodium. C) sodium gains an electron from chlorine. D) sodium and chlorine share an electron pair. E) chlorine gains an electron from sodium.

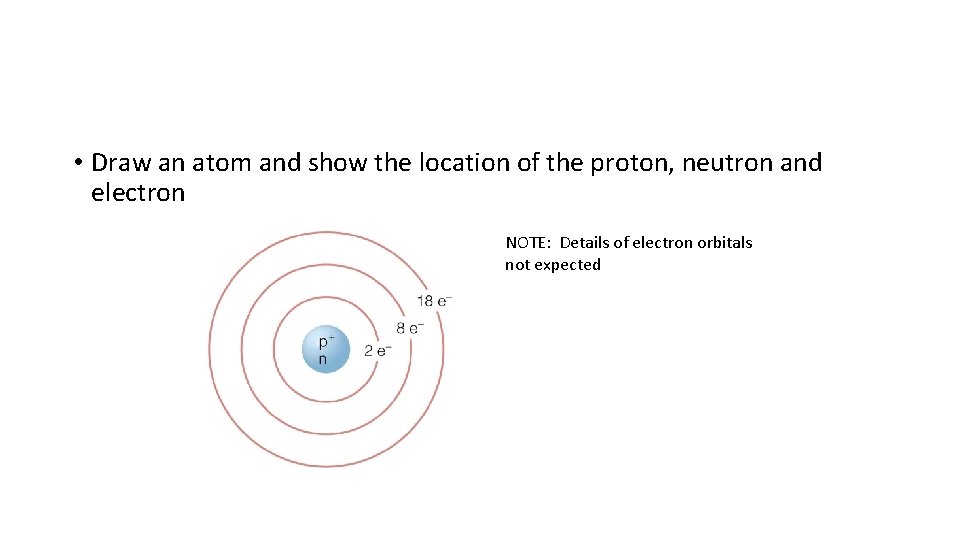
• Draw an atom and show the location of the proton, neutron and electron

• Draw an atom and show the location of the proton, neutron and electron NOTE: Details of electron orbitals not expected

What type of bond results from attraction of opposite charges?

What type of bond results from attraction of opposite charges? IONIC

Water, Everywhere Water is important because: • Most organisms have high water content (75 - 95%). • Many organisms live in water. • Most chemical reactions of life take place in water.

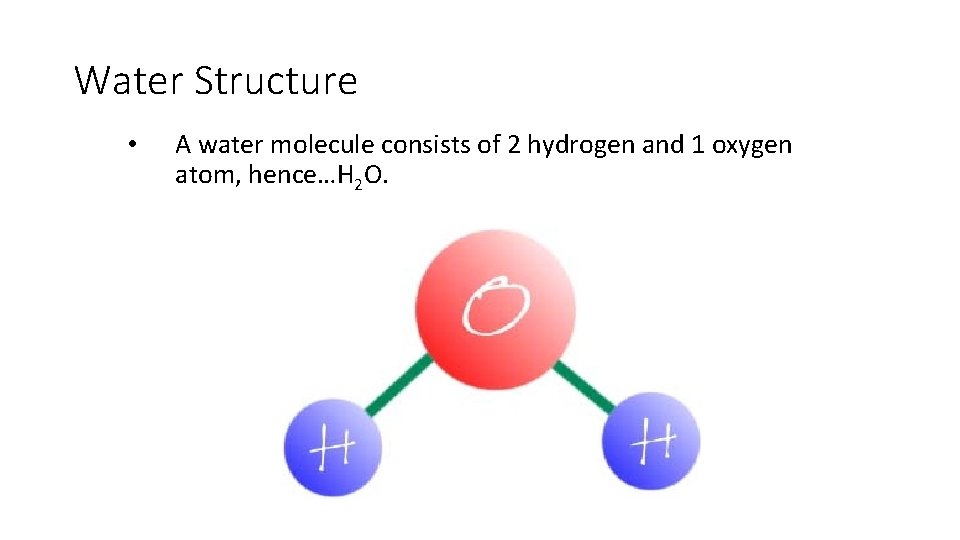
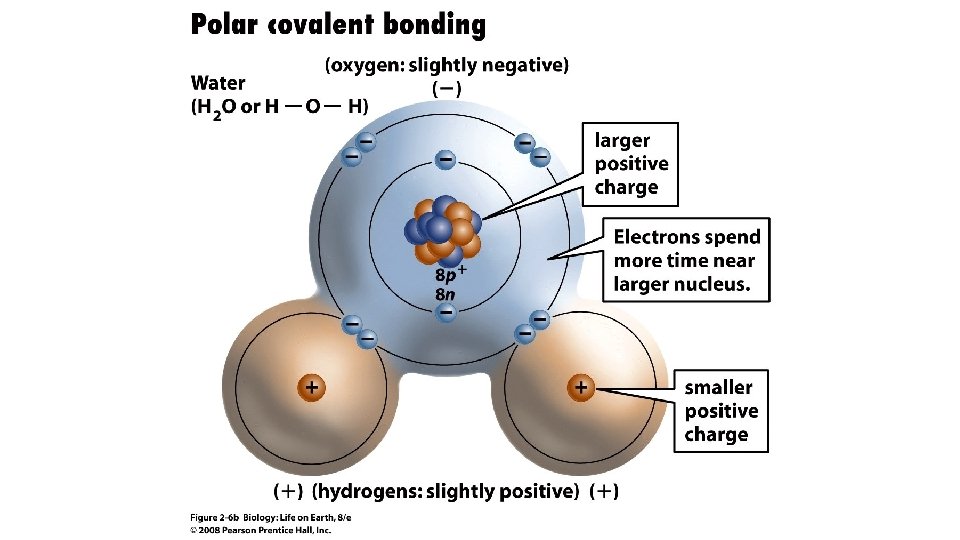
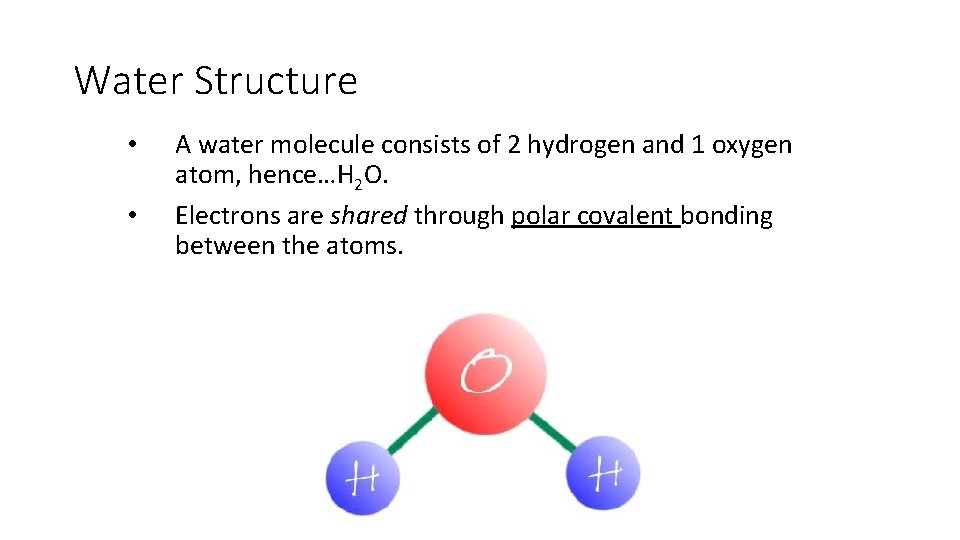
Water Structure • A water molecule consists of 2 hydrogen and 1 oxygen atom, hence…H 2 O.


Water Structure • • A water molecule consists of 2 hydrogen and 1 oxygen atom, hence…H 2 O. Electrons are shared through polar covalent bonding between the atoms.

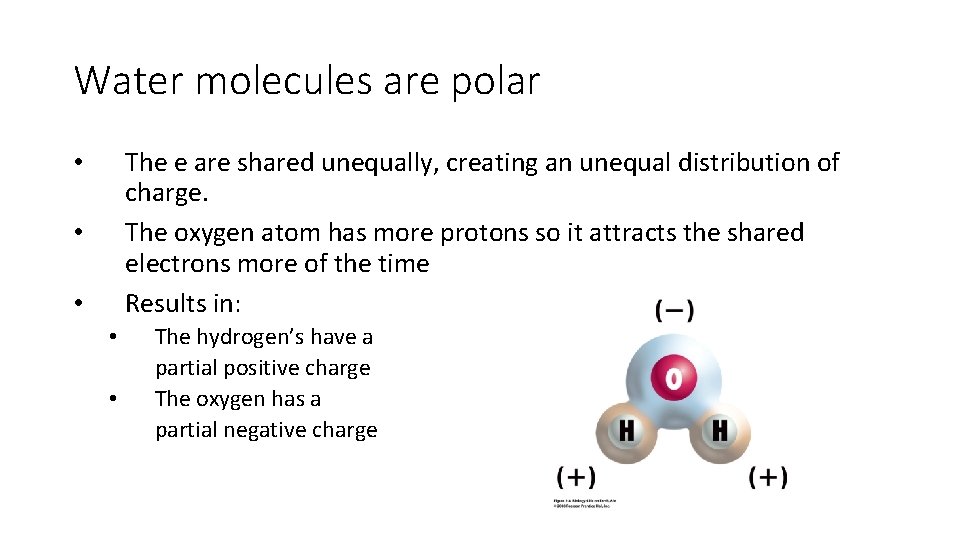
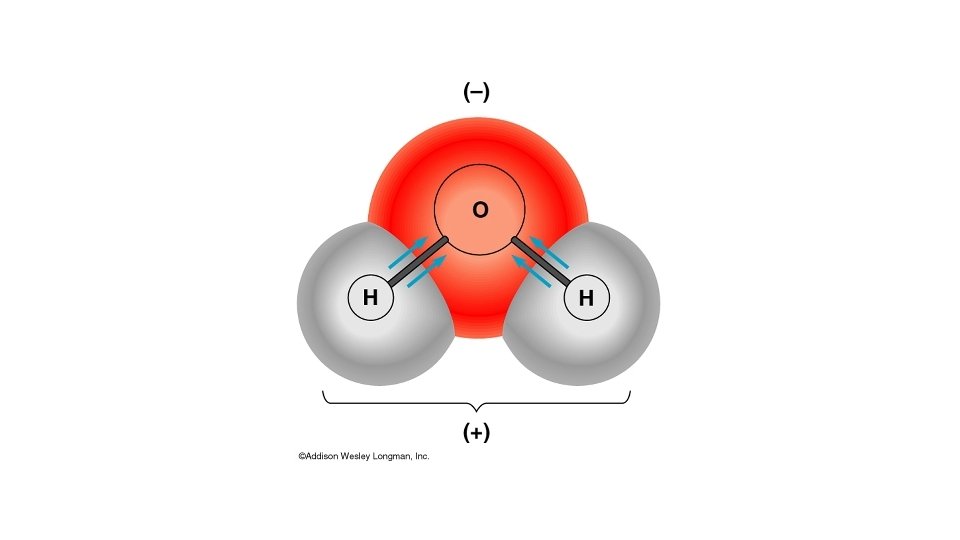
Water molecules are polar The e are shared unequally, creating an unequal distribution of charge. The oxygen atom has more protons so it attracts the shared electrons more of the time Results in: • • • The hydrogen’s have a partial positive charge The oxygen has a partial negative charge

• Draw water and show the polarity of the molecule



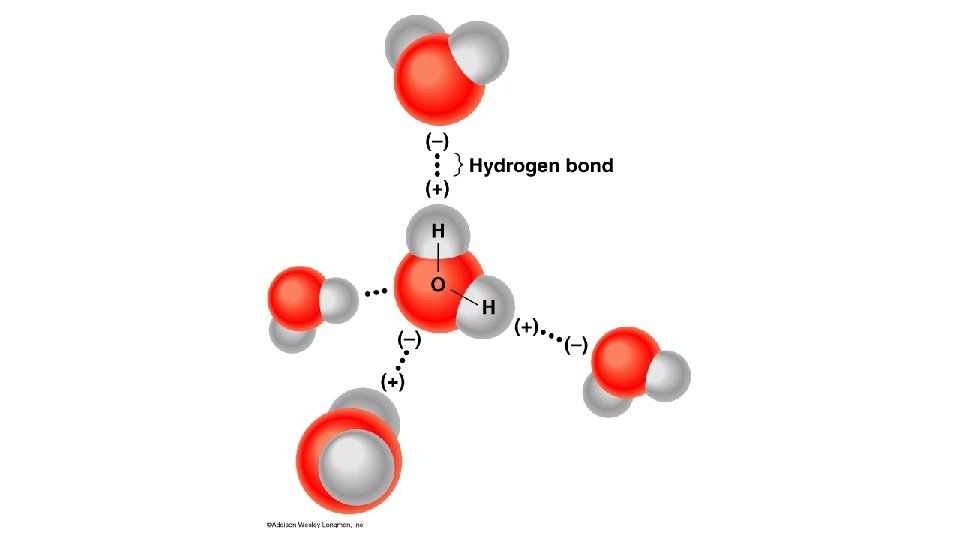
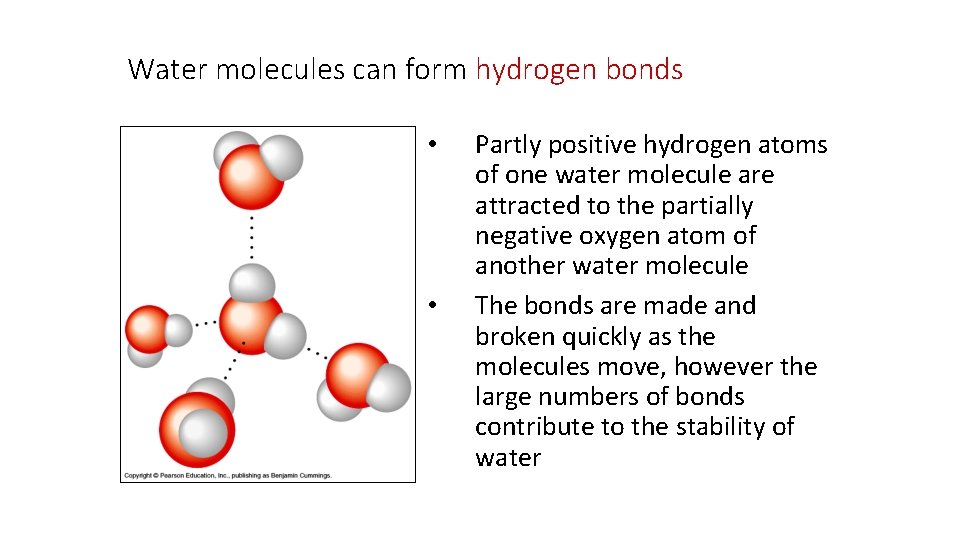
Water molecules can form hydrogen bonds • • Partly positive hydrogen atoms of one water molecule are attracted to the partially negative oxygen atom of another water molecule The bonds are made and broken quickly as the molecules move, however the large numbers of bonds contribute to the stability of water

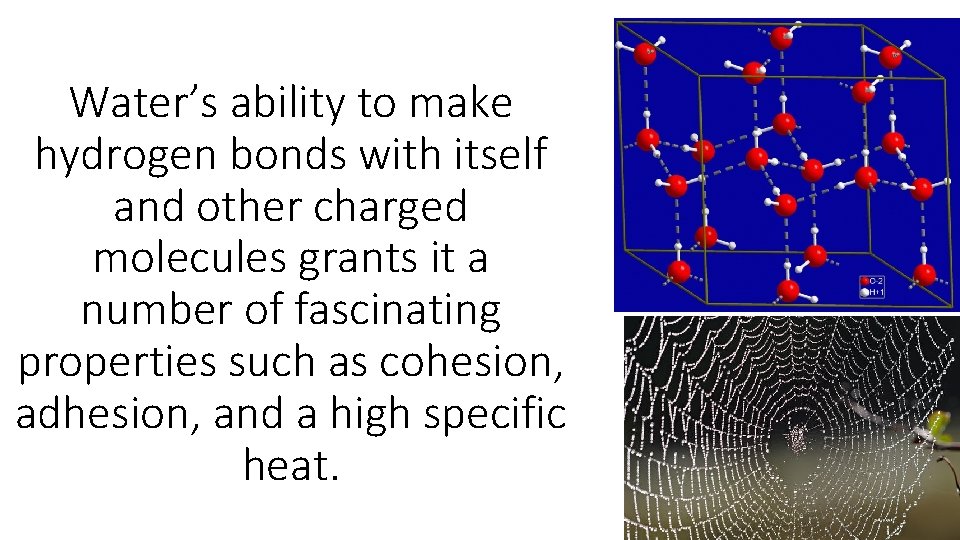
Water’s ability to make hydrogen bonds with itself and other charged molecules grants it a number of fascinating properties such as cohesion, adhesion, and a high specific heat.

Hydrogen bonds however aren’t something we can directly observe. They really are just a proposed explanation for why water has these remarkable properties. Because of this, hydrogen bonding is considered a theory.

The existence of hydrogen bonds will (probably) never be proven without a doubt. That doesn’t mean theory isn’t credible. We still have lots of evidence that our theory of H-Bonds is the best explanation we currently have for the properties of water.
