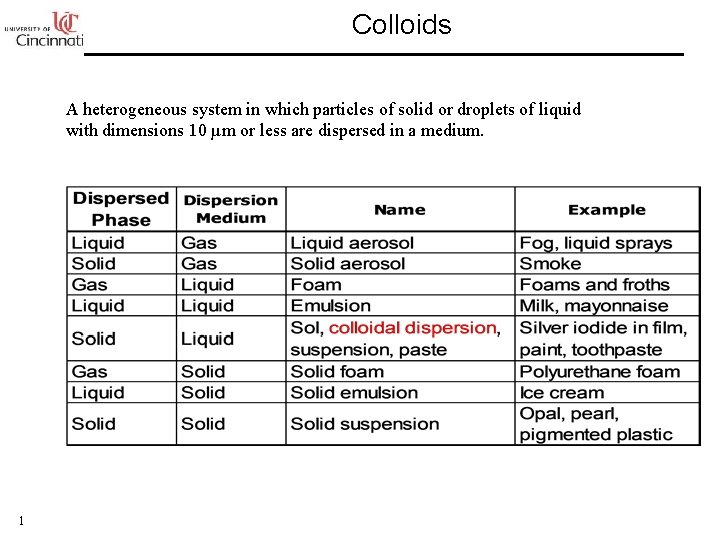
Colloids A heterogeneous system in which particles of







- Slides: 87

Colloids A heterogeneous system in which particles of solid or droplets of liquid with dimensions 10 µm or less are dispersed in a medium. 1

Properties of Colloidal Dispersions Very high surface area: 1 gal paint: 1 kg, 200 nm spheres 15, 000 m 2 Interfacial Energy Homework problem 1 Stability: Creaming, precipitation, coagulation, floculation (reversible) What is the relationship between colloid stability and intermolecular forces? 2

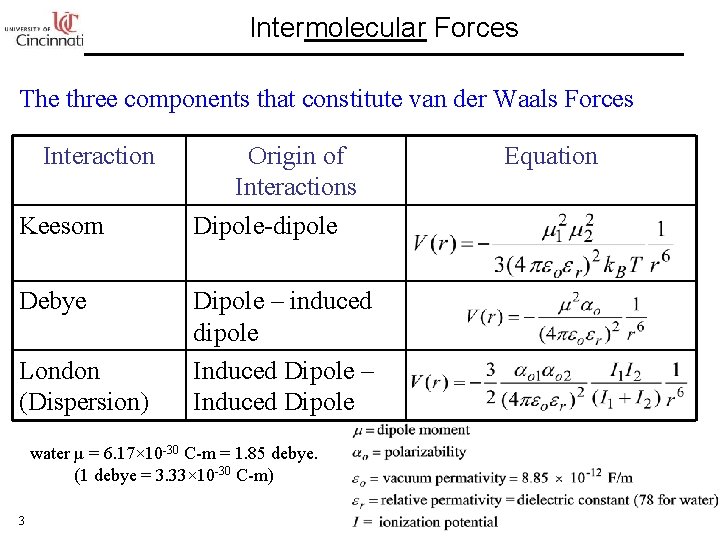
Intermolecular Forces The three components that constitute van der Waals Forces Interaction Keesom Debye London (Dispersion) Origin of Interactions Dipole-dipole Dipole – induced dipole Induced Dipole – Induced Dipole water µ = 6. 17× 10 -30 C-m = 1. 85 debye. (1 debye = 3. 33× 10 -30 C-m) 3 Equation

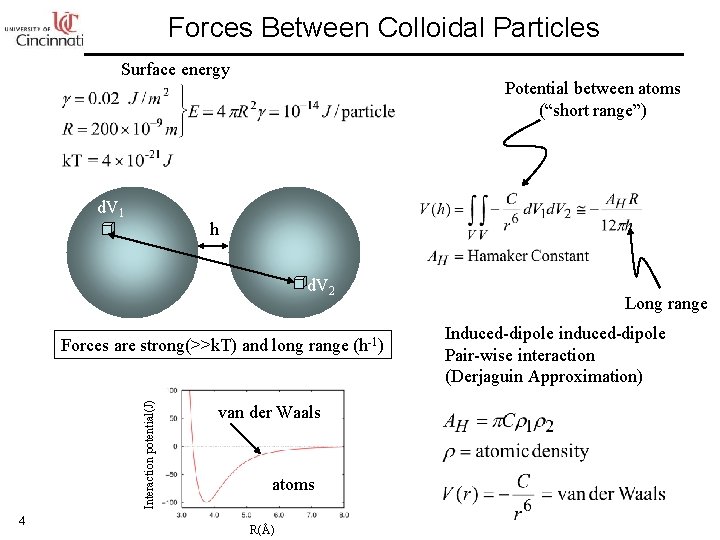
Forces Between Colloidal Particles Surface energy d. V 1 Potential between atoms (“short range”) h d. V 2 Interaction potential(J) Forces are strong(>>k. T) and long range (h-1) 4 van der Waals atoms R(Å) Long range Induced-dipole induced-dipole Pair-wise interaction (Derjaguin Approximation)

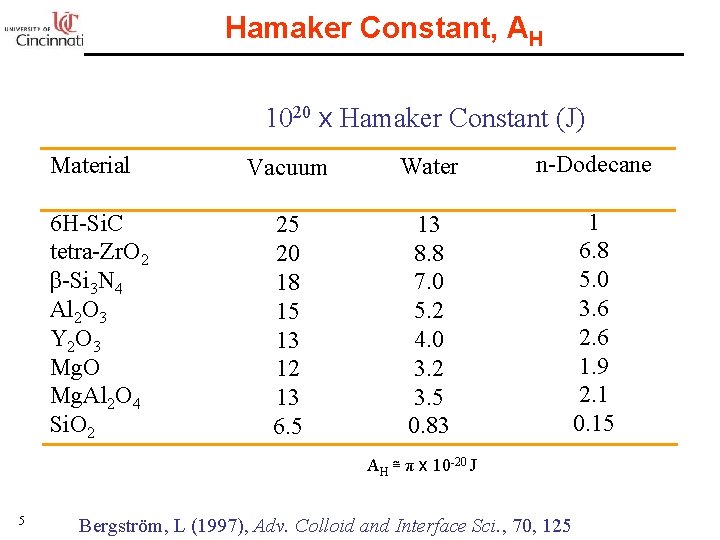
Hamaker Constant, AH 1020 x Hamaker Constant (J) Material 6 H-Si. C tetra-Zr. O 2 β-Si 3 N 4 Al 2 O 3 Y 2 O 3 Mg. O Mg. Al 2 O 4 Si. O 2 Vacuum Water n-Dodecane 25 20 18 15 13 12 13 6. 5 13 8. 8 7. 0 5. 2 4. 0 3. 2 3. 5 0. 83 1 6. 8 5. 0 3. 6 2. 6 1. 9 2. 1 0. 15 AH ≅ π x 10 -20 J 5 Bergström, L (1997), Adv. Colloid and Interface Sci. , 70, 125

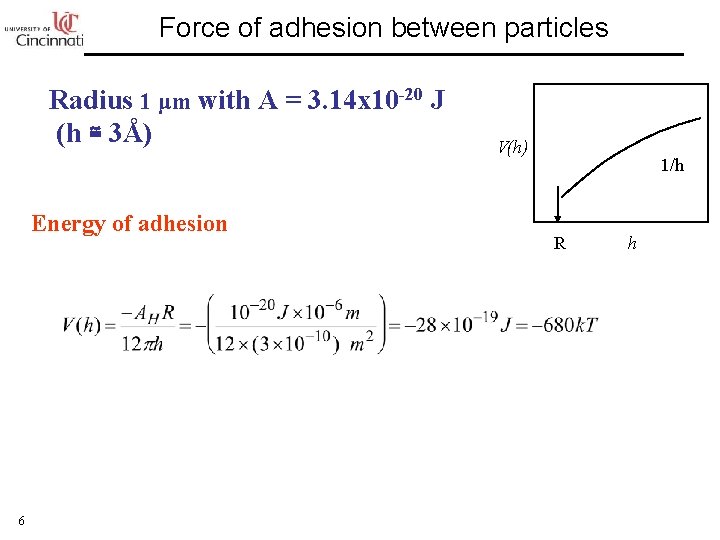
Force of adhesion between particles Radius 1 μm with A = 3. 14 x 10 -20 J (h ≅ 3Å) Energy of adhesion 6 V(h) 1/h R h

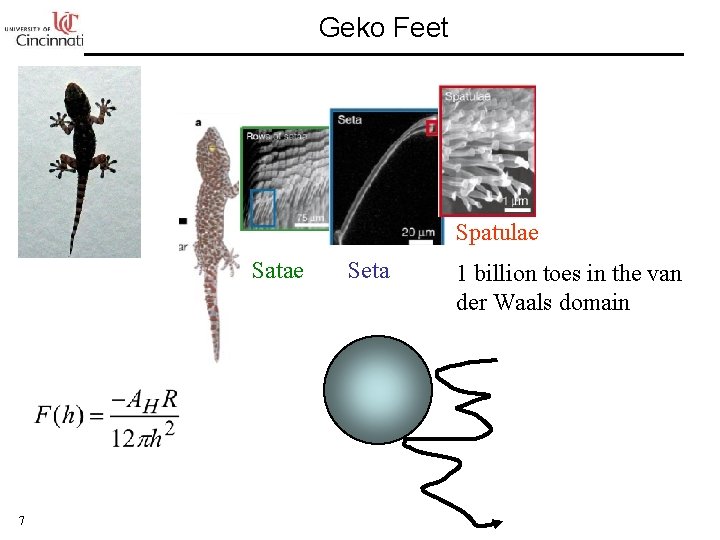
Geko Feet Spatulae Satae 7 Seta 1 billion toes in the van der Waals domain

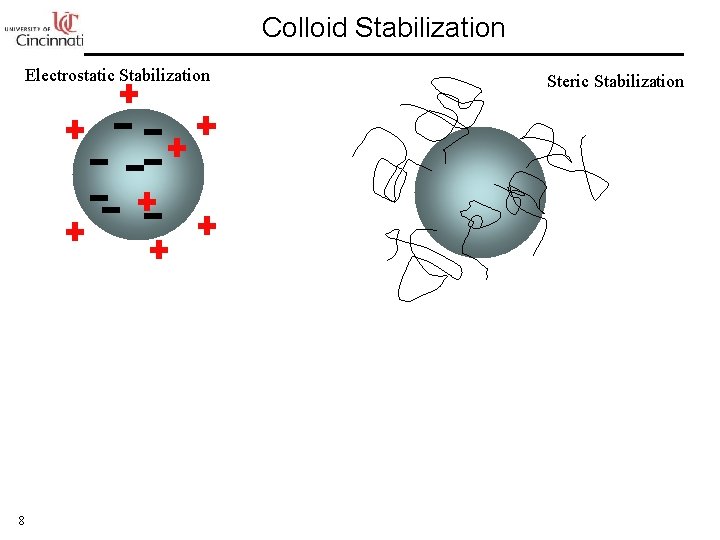
Colloid Stabilization Electrostatic Stabilization 8 Steric Stabilization

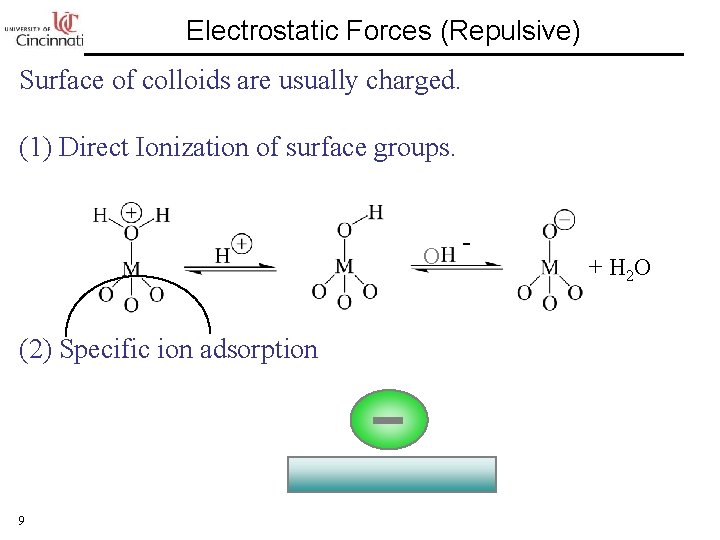
Electrostatic Forces (Repulsive) Surface of colloids are usually charged. (1) Direct Ionization of surface groups. O (2) Specific ion adsorption 9 - + H 2 O

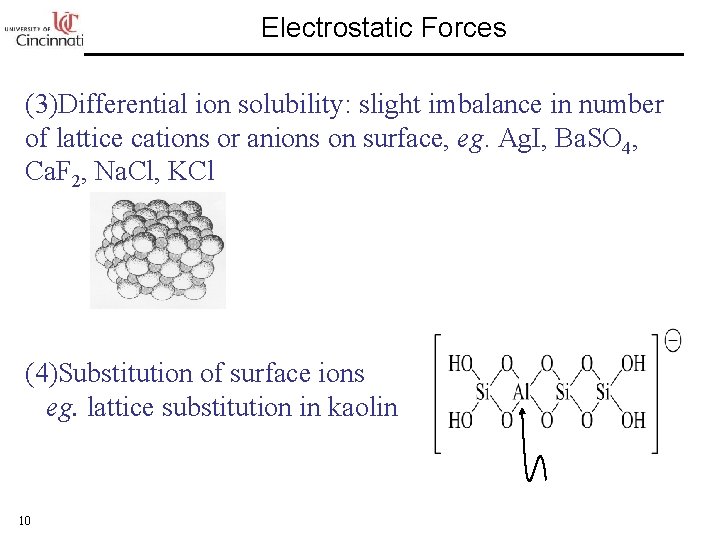
Electrostatic Forces (3)Differential ion solubility: slight imbalance in number of lattice cations or anions on surface, eg. Ag. I, Ba. SO 4, Ca. F 2, Na. Cl, KCl (4)Substitution of surface ions eg. lattice substitution in kaolin 10

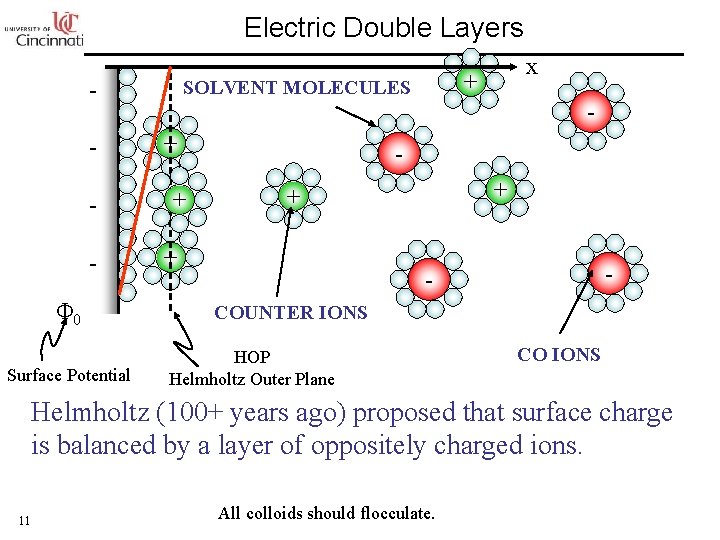
Electric Double Layers Φ 0 Surface Potential + SOLVENT MOLECULES - x - + + + - COUNTER IONS HOP Helmholtz Outer Plane CO IONS Helmholtz (100+ years ago) proposed that surface charge is balanced by a layer of oppositely charged ions. 11 All colloids should flocculate.

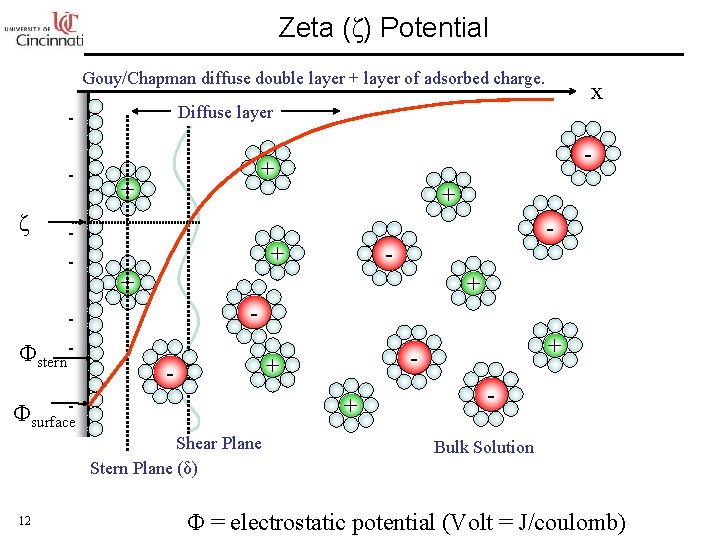
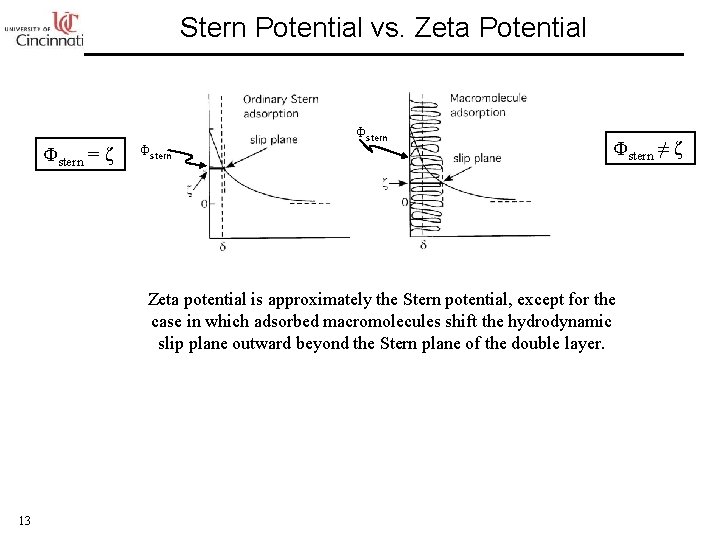
Zeta (ζ) Potential Gouy/Chapman diffuse double layer + layer of adsorbed charge. Diffuse layer ζ - Φstern - - + + - + Φsurface Shear Plane Stern Plane (δ) + - - 12 x Bulk Solution Φ = electrostatic potential (Volt = J/coulomb)

Stern Potential vs. Zeta Potential Φstern = ζ Φstern ≠ ζ Zeta potential is approximately the Stern potential, except for the case in which adsorbed macromolecules shift the hydrodynamic slip plane outward beyond the Stern plane of the double layer. 13

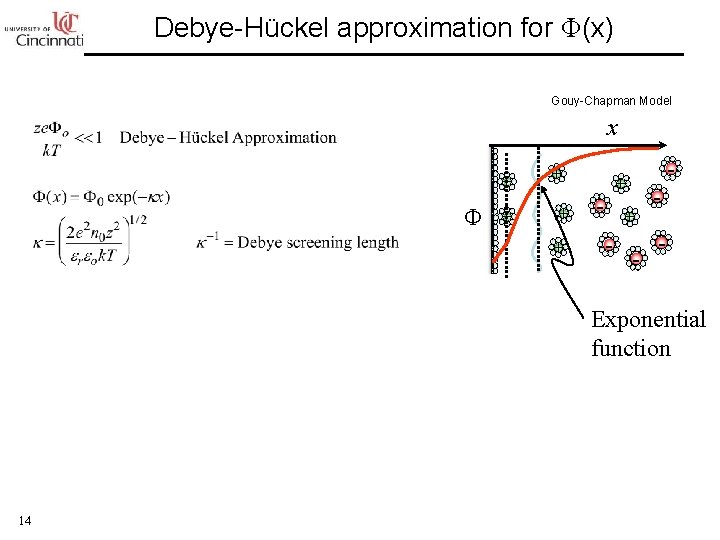
Debye-Hückel approximation for Φ(x) Gouy-Chapman Model x + Φ + + + - - Exponential function 14

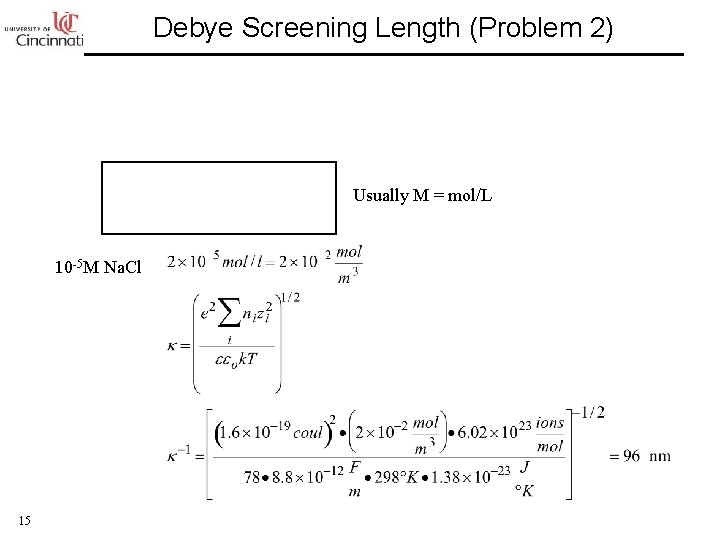
Debye Screening Length (Problem 2) Usually M = mol/L 10 -5 M Na. Cl 15

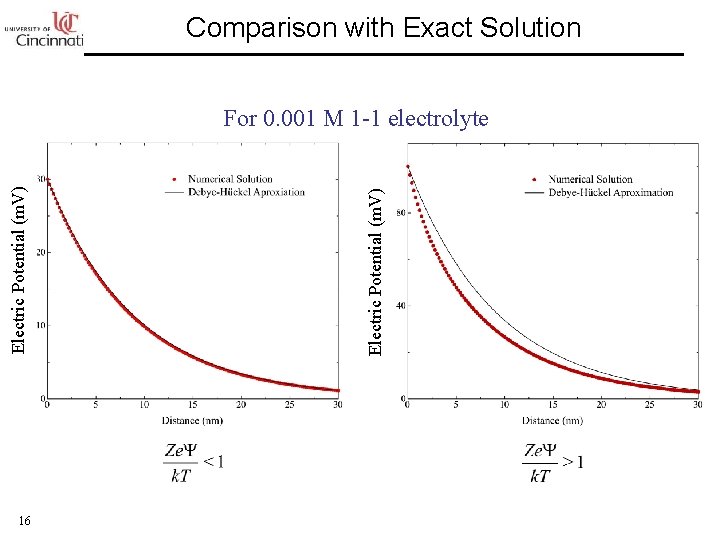
Comparison with Exact Solution 16 Electric Potential (m. V) For 0. 001 M 1 -1 electrolyte

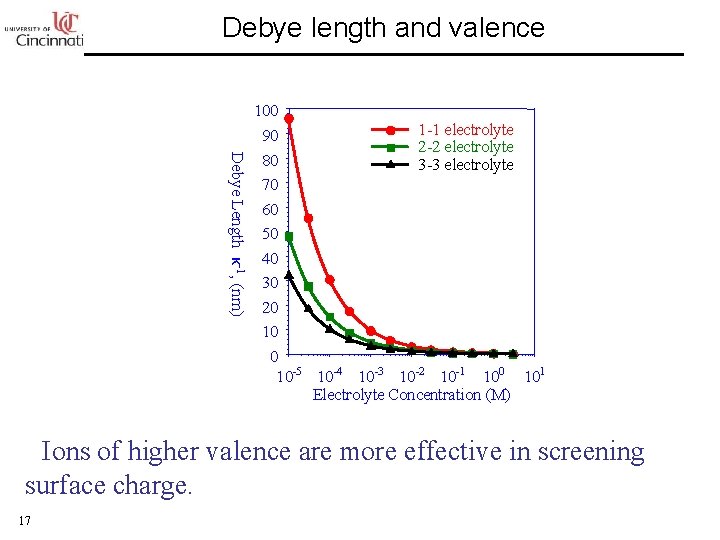
Debye length and valence Debye Length κ-1, (nm) 100 1 -1 electrolyte 90 2 -2 electrolyte 80 3 -3 electrolyte 70 60 50 40 30 20 10 -5 10 -4 10 -3 10 -2 10 -1 100 101 Electrolyte Concentration (M) • Ions of higher valence are more effective in screening surface charge. 17

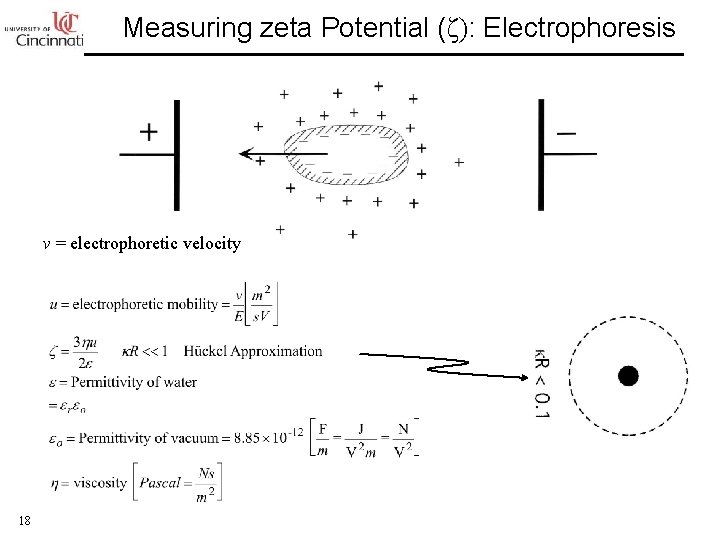
Measuring zeta Potential (ζ): Electrophoresis v = electrophoretic velocity 18

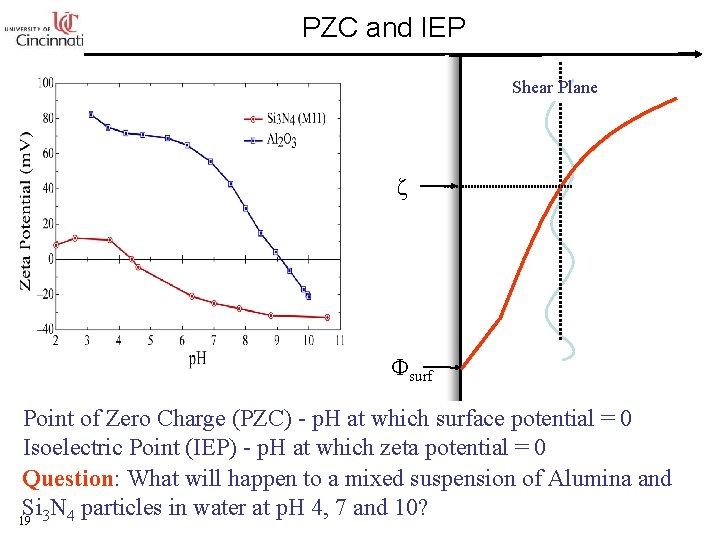
PZC and IEP Shear Plane ζ Φsurf Point of Zero Charge (PZC) - p. H at which surface potential = 0 Isoelectric Point (IEP) - p. H at which zeta potential = 0 Question: What will happen to a mixed suspension of Alumina and Si N particles in water at p. H 4, 7 and 10? 19 3 4

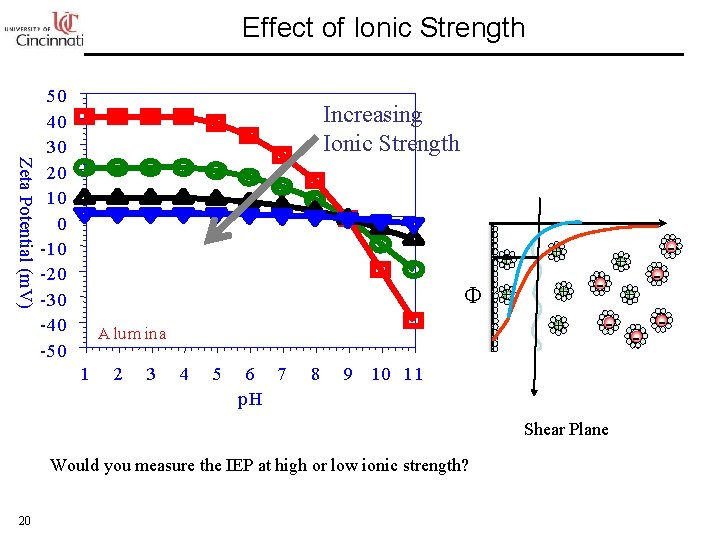
Effect of Ionic Strength Zeta Potential (m. V) 50 40 30 20 10 0 -10 -20 -30 -40 -50 Increasing Ionic Strength + Φ + A lum ina 1 2 3 4 5 6 7 p. H 8 + + - + - 9 10 11 Shear Plane Would you measure the IEP at high or low ionic strength? 20 -

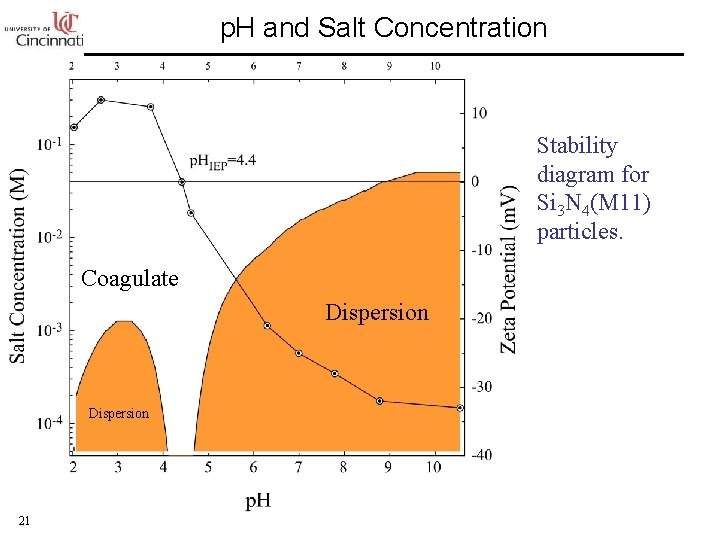
p. H and Salt Concentration Stability diagram for Si 3 N 4(M 11) particles. Coagulate Dispersion 21

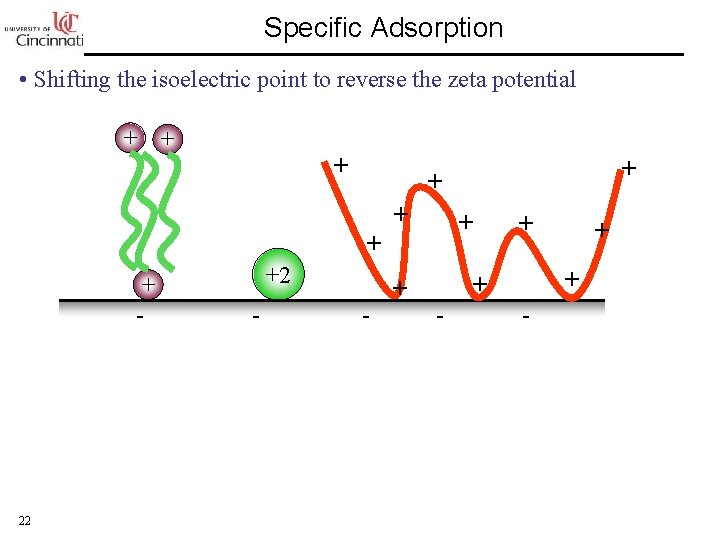
Specific Adsorption • Shifting the isoelectric point to reverse the zeta potential + + + +2 + - 22 - + + + + -

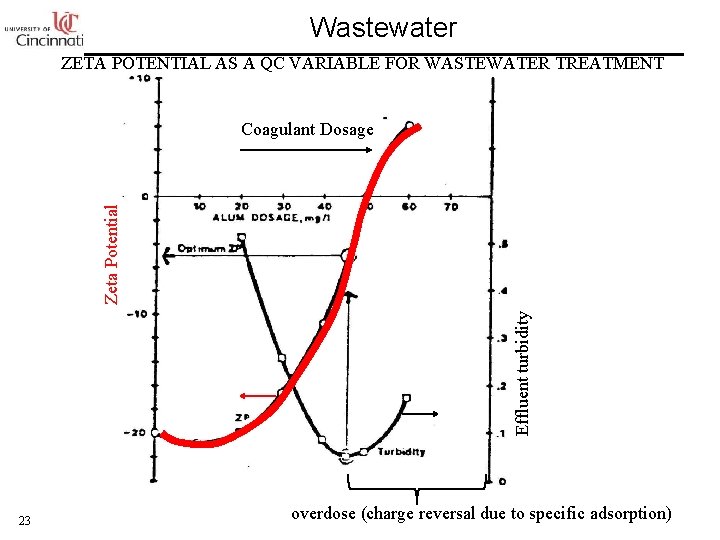
Wastewater ZETA POTENTIAL AS A QC VARIABLE FOR WASTEWATER TREATMENT Effluent turbidity Zeta Potential Coagulant Dosage 23 overdose (charge reversal due to specific adsorption)

24 8/2 M Colloids II Schaefer 8/4 W Colloids III Schaefer 8/9 M Quiz 4 Polymer Properties I C 15 Schaefer C 532 -561 8/11 W Polymer Properties II C 16 Schaefer C 570 -587 8/16 M Polymer Properties III C 16 Schaefer C 588 -607 8/18 W Stainless Steel and Corrosion C 14 Schaefer C 673 - 690 8/23 M Corrosion and electrochem C 14 Schaefer C 691 -706 8/25 W Quiz 5 Corrosion Prevention C 14 Schaefer 9/1 W Final 10: 30 -12: 30 p. m.

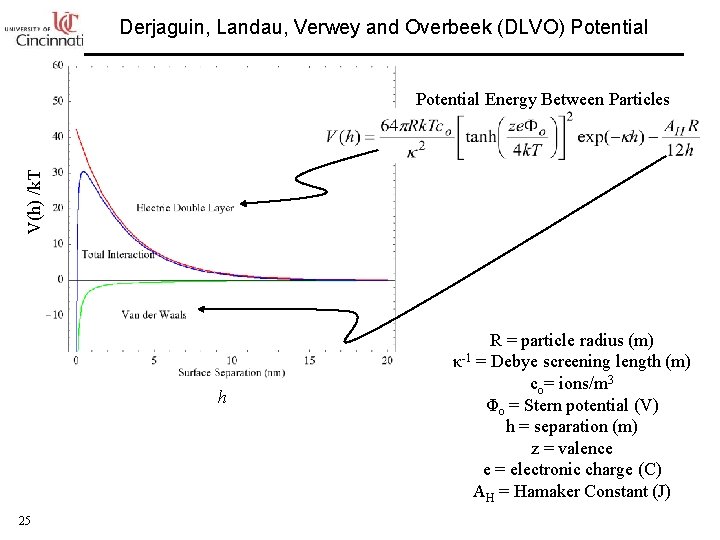
Derjaguin, Landau, Verwey and Overbeek (DLVO) Potential V(h) /k. T Potential Energy Between Particles h 25 R = particle radius (m) κ-1 = Debye screening length (m) co= ions/m 3 Φo = Stern potential (V) h = separation (m) z = valence e = electronic charge (C) AH = Hamaker Constant (J)

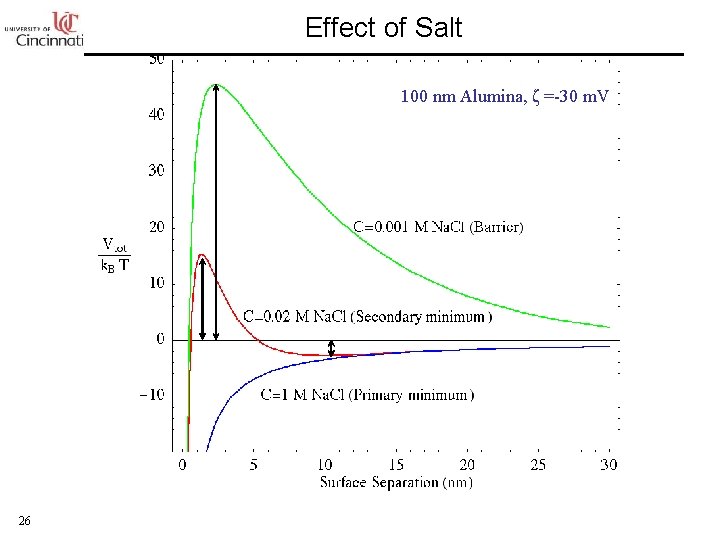
Effect of Salt 100 nm Alumina, ζ =-30 m. V 26

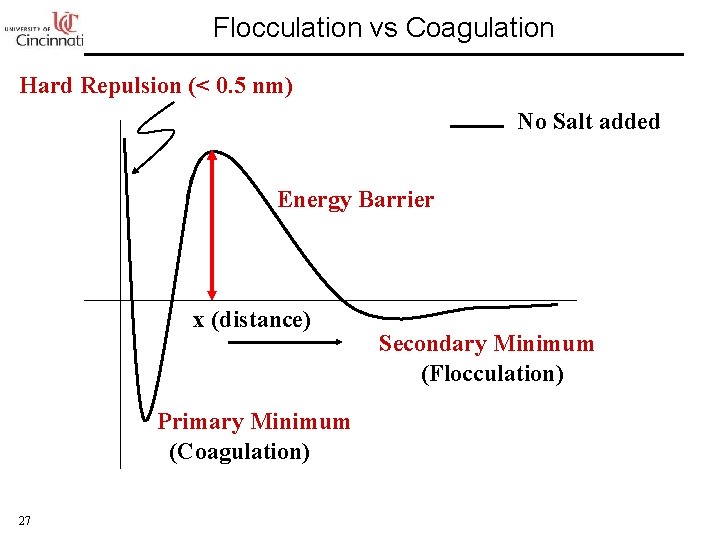
Flocculation vs Coagulation Hard Repulsion (< 0. 5 nm) No Salt added Energy Barrier x (distance) Primary Minimum (Coagulation) 27 Secondary Minimum (Flocculation)

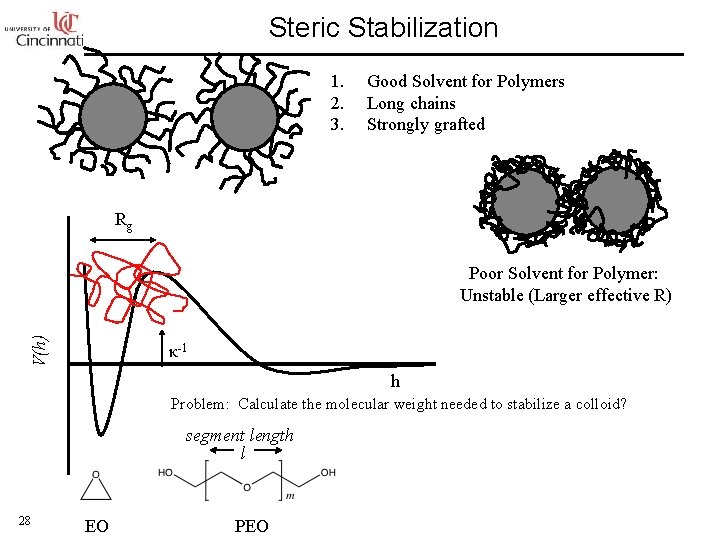
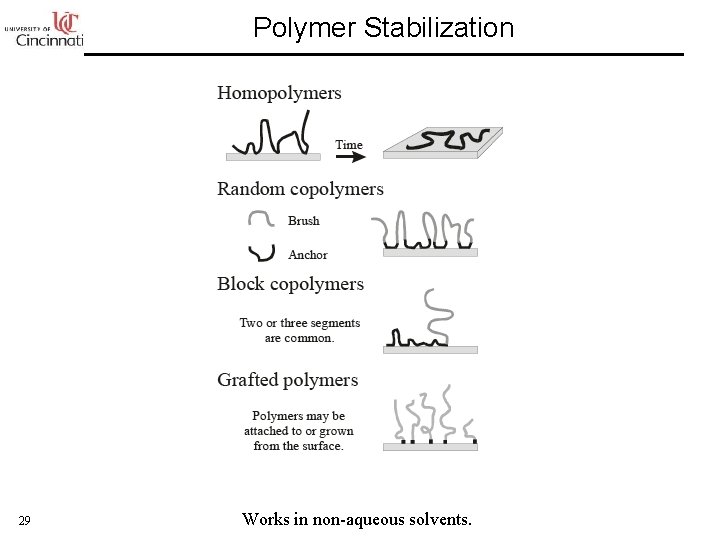
Steric Stabilization 1. 2. 3. Good Solvent for Polymers Long chains Strongly grafted Rg V(h) Poor Solvent for Polymer: Unstable (Larger effective R) κ-1 h Problem: Calculate the molecular weight needed to stabilize a colloid? segment length l 28 EO PEO

Polymer Stabilization 29 Works in non-aqueous solvents.

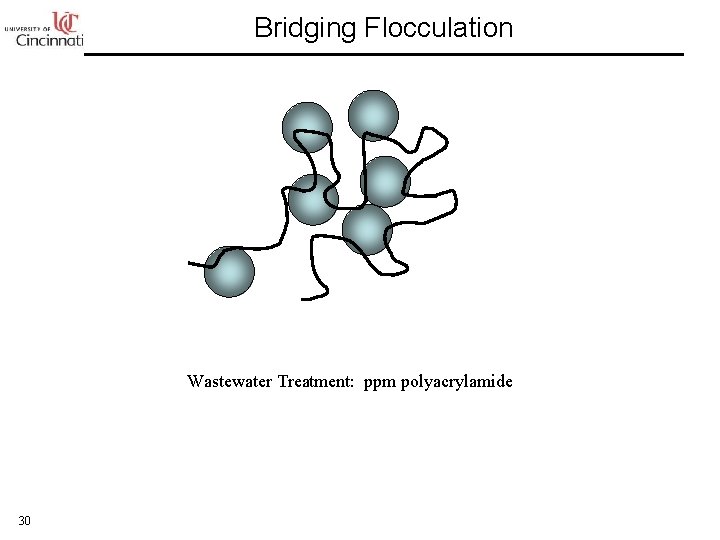
Bridging Flocculation Wastewater Treatment: ppm polyacrylamide 30

31

Synthesis of Colloids 32

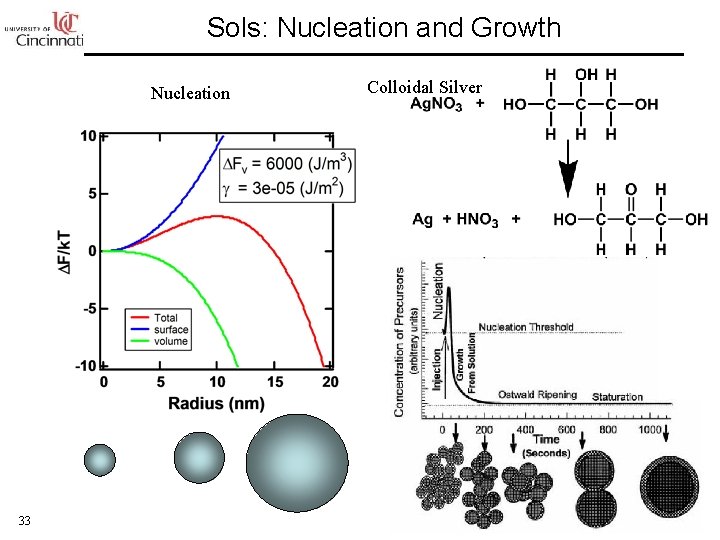
Sols: Nucleation and Growth Nucleation 33 Colloidal Silver

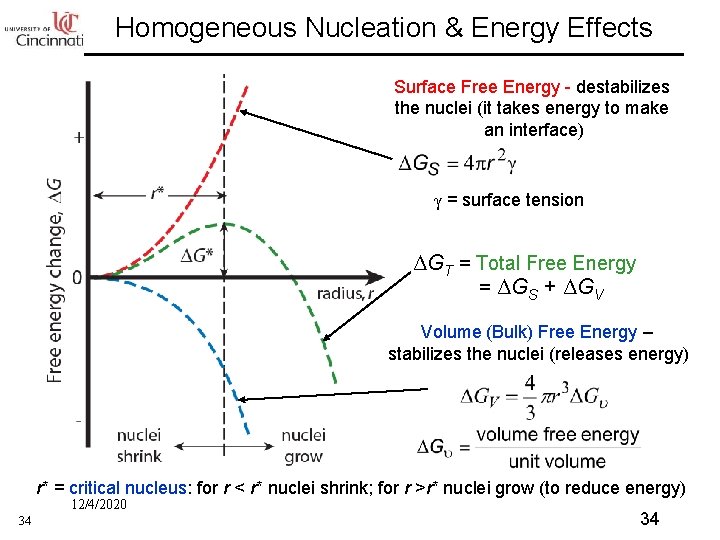
Homogeneous Nucleation & Energy Effects Surface Free Energy - destabilizes the nuclei (it takes energy to make an interface) γ = surface tension ΔGT = Total Free Energy = ΔGS + ΔGV Volume (Bulk) Free Energy – stabilizes the nuclei (releases energy) r* = critical nucleus: for r < r* nuclei shrink; for r >r* nuclei grow (to reduce energy) 12/4/2020 34 34

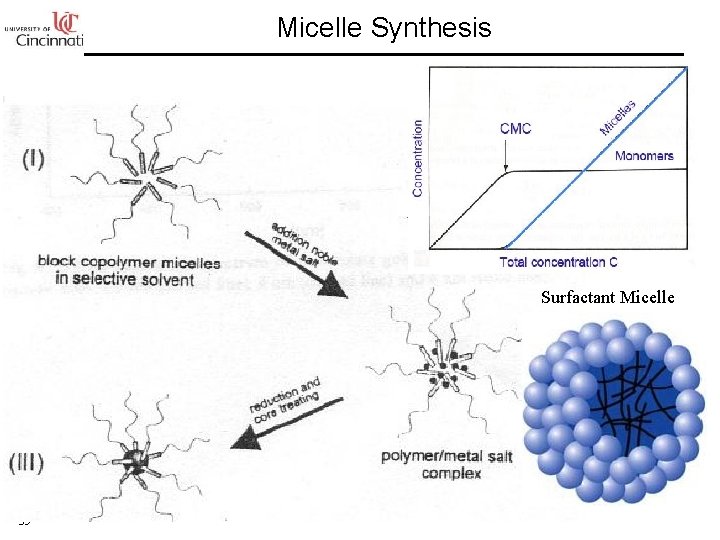
Micelle Synthesis Surfactant Micelle 35

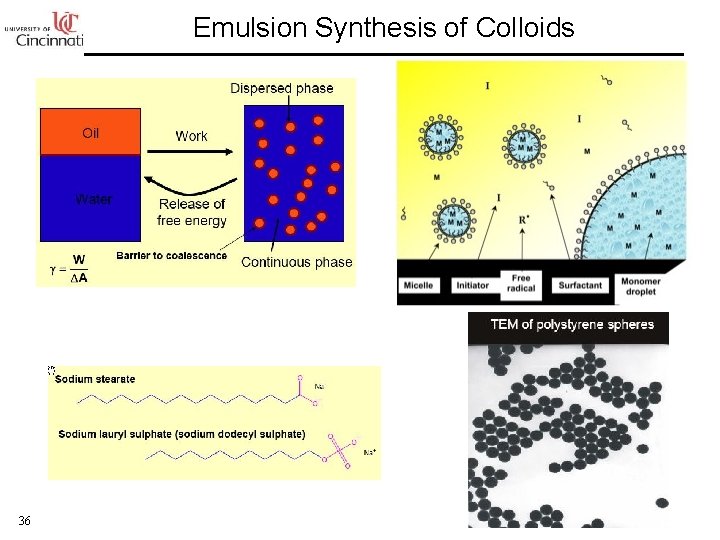
Emulsion Synthesis of Colloids 36

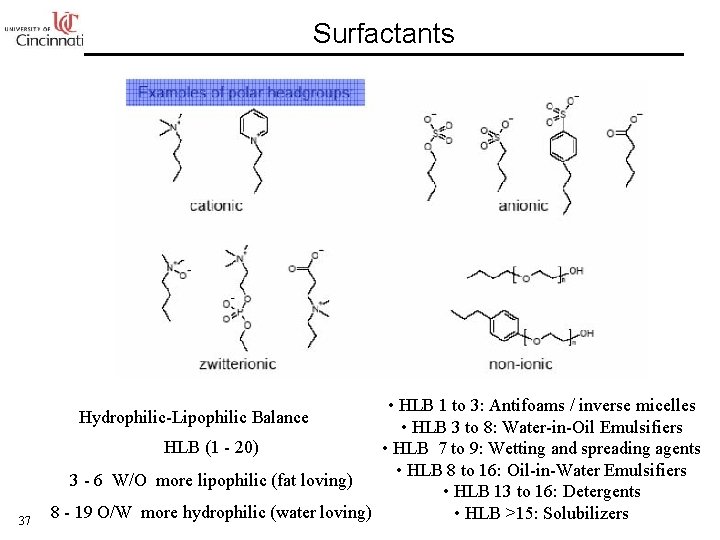
Surfactants • HLB 1 to 3: Antifoams / inverse micelles • HLB 3 to 8: Water-in-Oil Emulsifiers HLB (1 - 20) • HLB 7 to 9: Wetting and spreading agents • HLB 8 to 16: Oil-in-Water Emulsifiers 3 - 6 W/O more lipophilic (fat loving) • HLB 13 to 16: Detergents 8 - 19 O/W more hydrophilic (water loving) • HLB >15: Solubilizers Hydrophilic-Lipophilic Balance 37

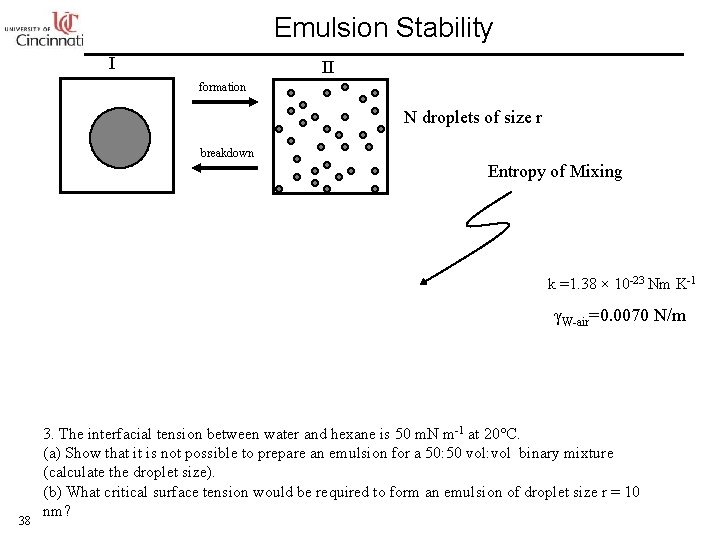
Emulsion Stability I II formation N droplets of size r breakdown Entropy of Mixing k =1. 38 × 10 -23 Nm K-1 γW-air=0. 0070 N/m 38 3. The interfacial tension between water and hexane is 50 m. N m-1 at 20°C. (a) Show that it is not possible to prepare an emulsion for a 50: 50 vol: vol binary mixture (calculate the droplet size). (b) What critical surface tension would be required to form an emulsion of droplet size r = 10 nm?

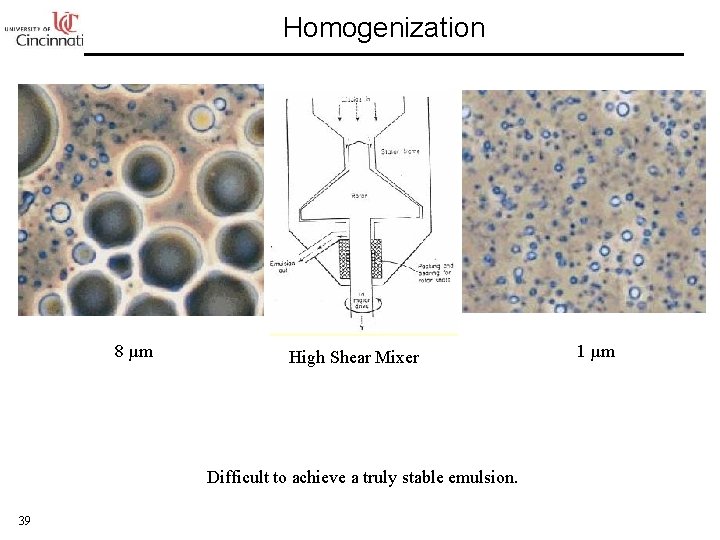
Homogenization 8 µm High Shear Mixer Difficult to achieve a truly stable emulsion. 39 1 µm

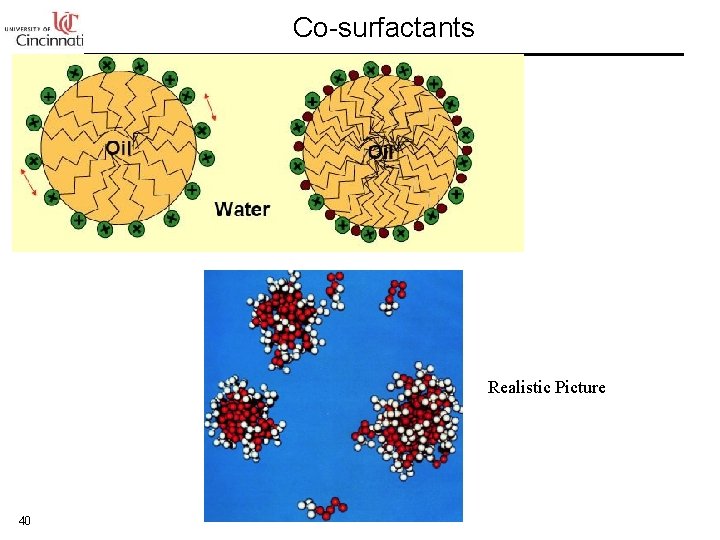
Co-surfactants Realistic Picture 40

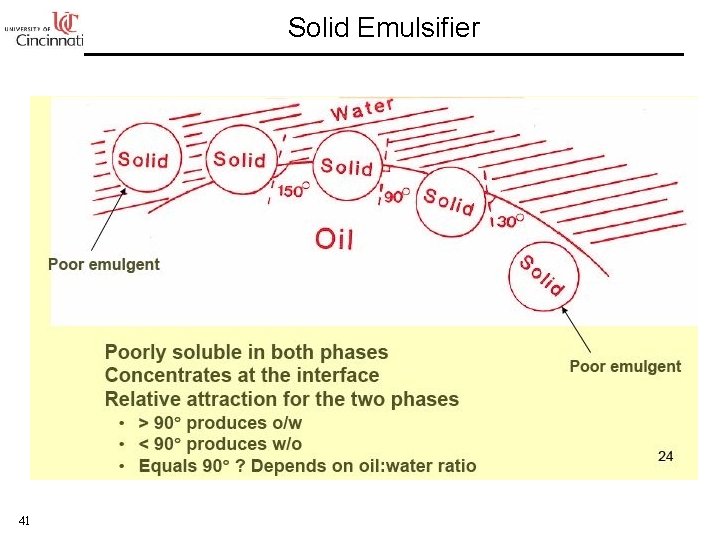
Solid Emulsifier 41

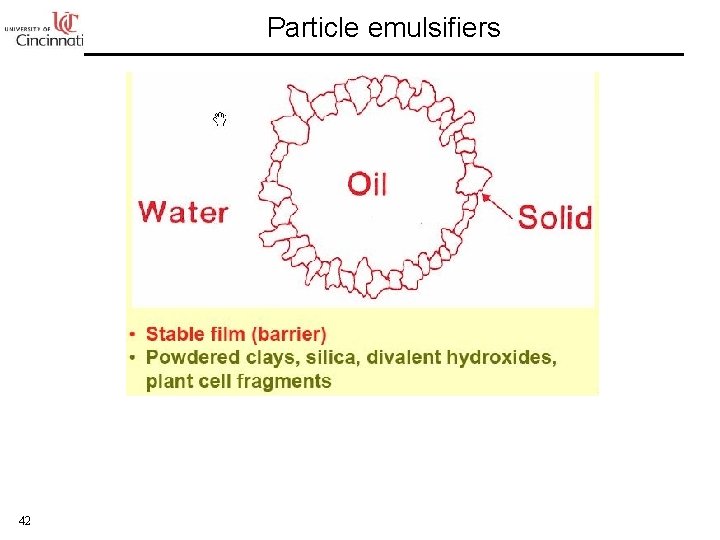
Particle emulsifiers 42

Water Drops Floating on Water http: //web. mac. com/drspam/i. Web/Alaska/Kvichak. html 43

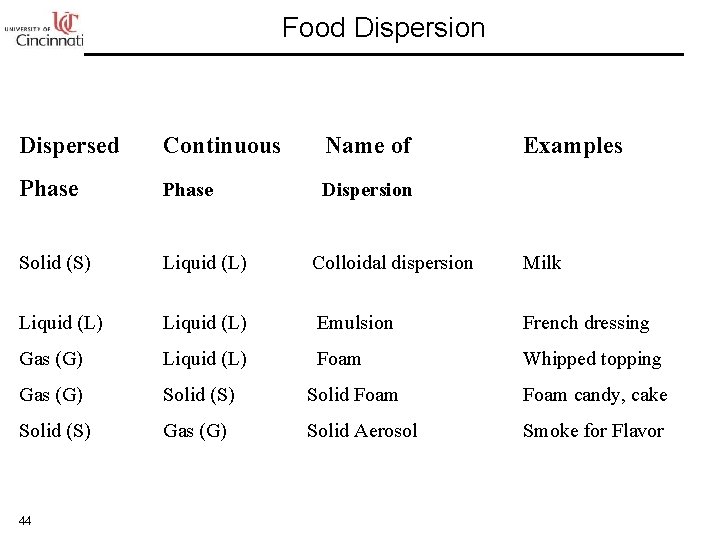
Food Dispersion Dispersed Continuous Name of Phase Dispersion Solid (S) Liquid (L) Colloidal dispersion Milk Liquid (L) Emulsion French dressing Gas (G) Liquid (L) Foam Whipped topping Gas (G) Solid (S) Solid Foam candy, cake Solid (S) Gas (G) Solid Aerosol Smoke for Flavor 44 Examples

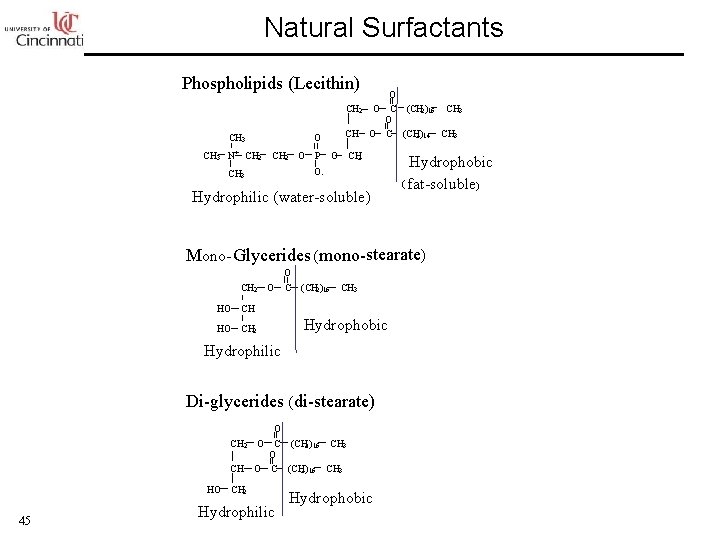
Natural Surfactants Phospholipids (Lecithin) CH 3 N+ O CH 2 O C (CH 2 )16 CH 3 O CH O C (CH 2 )14 CH 3 P O CH 2 O- CH 3 Hydrophilic (water-soluble) Hydrophobic ( fat-soluble) Mono- Glycerides (mono-stearate) CH 2 HO CH 2 O O C (CH 2 )16 CH 3 Hydrophobic Hydrophilic Di-glycerides ( di-stearate) O C (CH 2 )16 CH 3 O O C (CH 2 )16 CH 3 CH 2 O CH HO 45 CH 2 Hydrophilic Hydrophobic

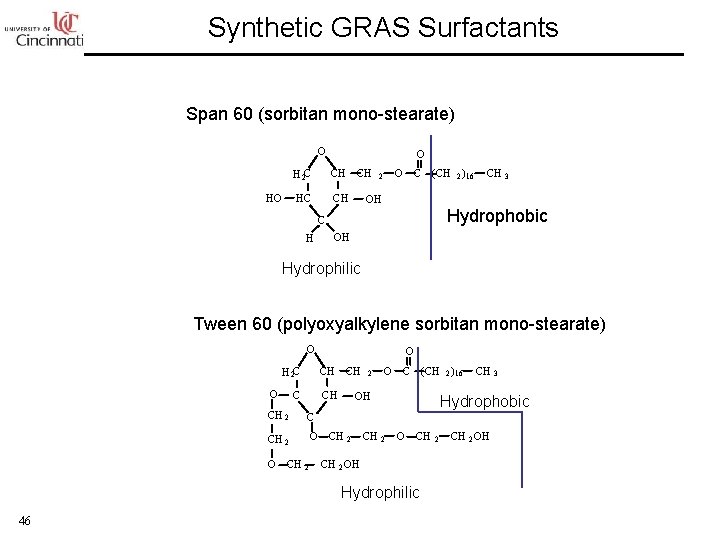
Synthetic GRAS Surfactants Span 60 (sorbitan mono-stearate) O HO O H 2 C CH CH HC CH O 2 C (CH 2 ) 16 OH Hydrophobic C H CH 3 OH Hydrophilic Tween 60 (polyoxyalkylene sorbitan mono-stearate) O H 2 C CH O CH 2 C CH 2 O O O CH 2 O C (CH 2 ) 16 OH CH 2 Hydrophobic O CH CH 2 OH Hydrophilic 46 CH 3 2 CH 2 OH

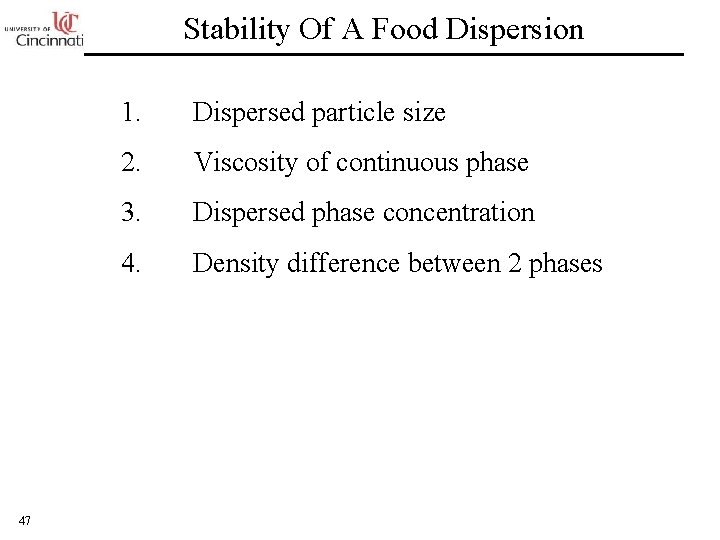
Stability Of A Food Dispersion 47 1. Dispersed particle size 2. Viscosity of continuous phase 3. Dispersed phase concentration 4. Density difference between 2 phases

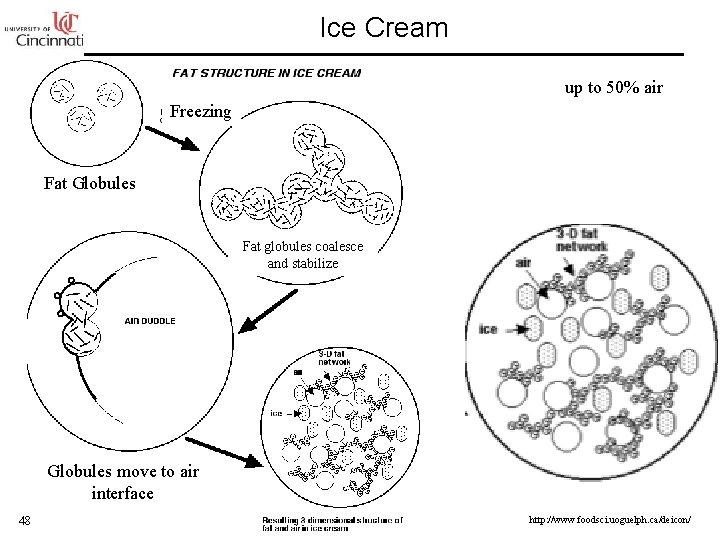
Ice Cream up to 50% air Freezing Fat Globules Fat globules coalesce and stabilize Globules move to air interface 48 http: //www. foodsci. uoguelph. ca/deicon/

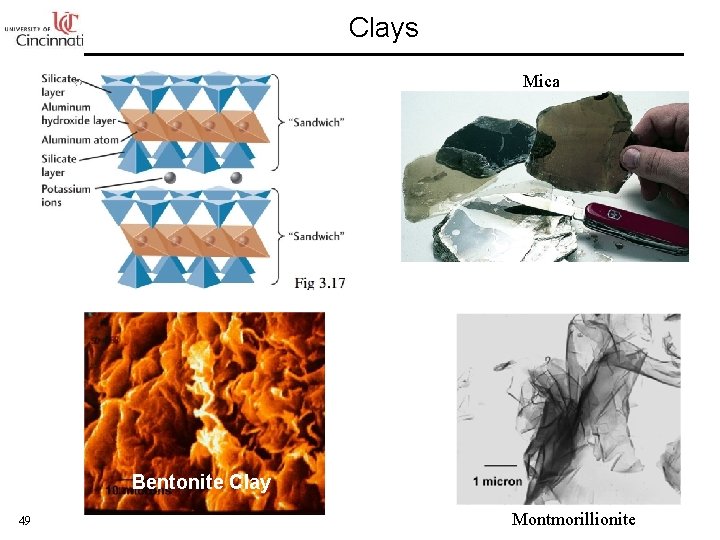
Clays Mica Bentonite Clay 49 Montmorillionite

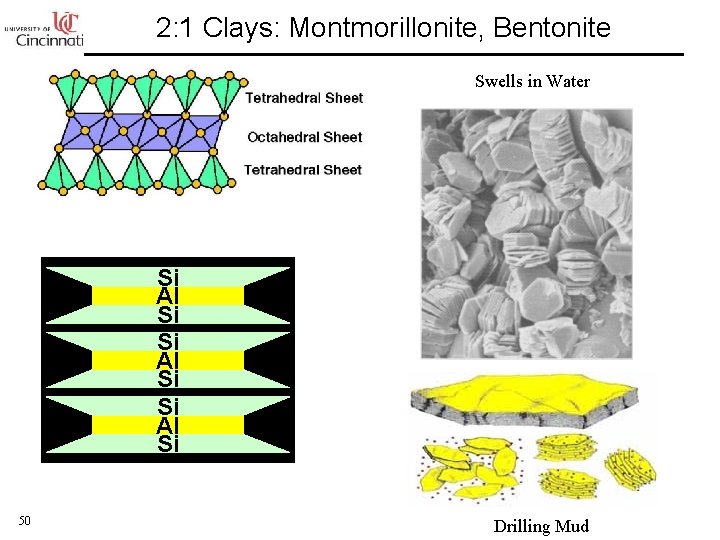
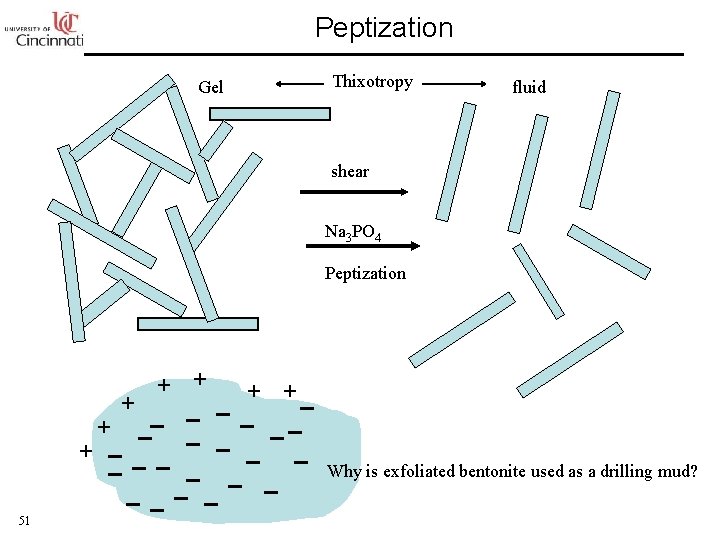
2: 1 Clays: Montmorillonite, Bentonite Swells in Water Si Al Si 50 Drilling Mud

Peptization Gel Thixotropy fluid shear Na 3 PO 4 Peptization + +_ + _ __ _ + _ _ _ _ 51 Why is exfoliated bentonite used as a drilling mud?

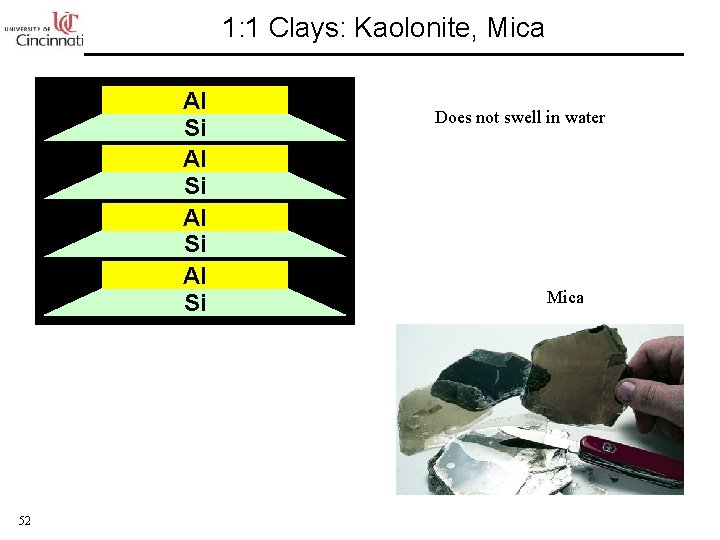
1: 1 Clays: Kaolonite, Mica Al Si 52 Does not swell in water Mica

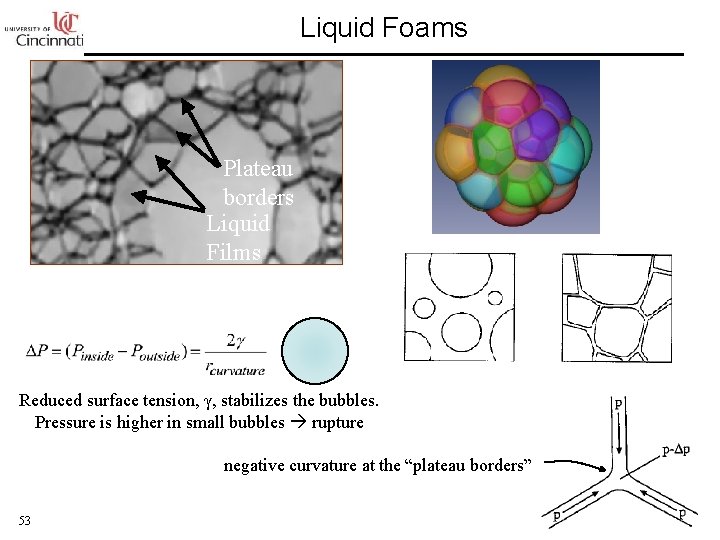
Liquid Foams Plateau borders Liquid Films Reduced surface tension, γ, stabilizes the bubbles. Pressure is higher in small bubbles rupture negative curvature at the “plateau borders” 53

54

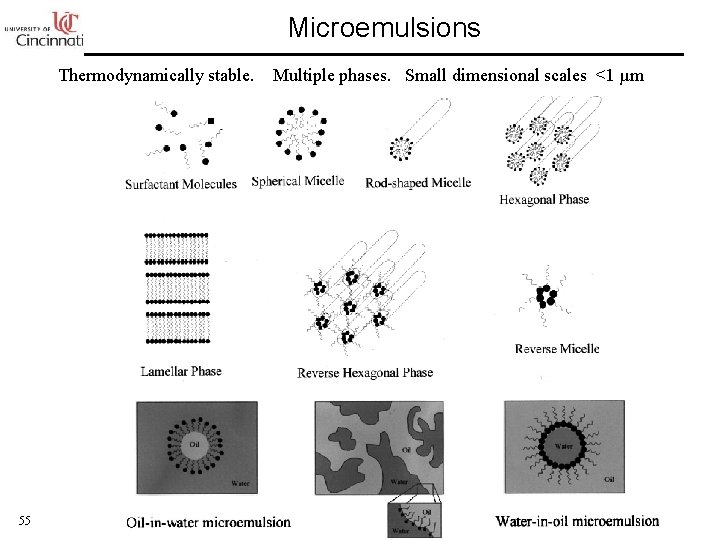
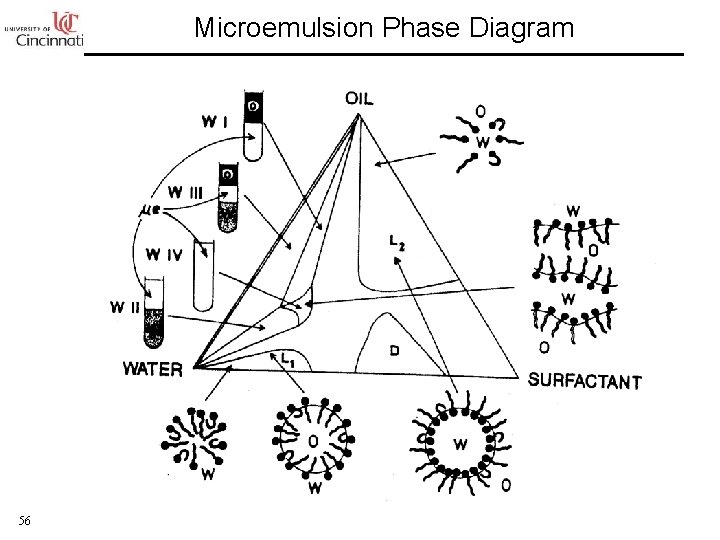
Microemulsions Thermodynamically stable. 55 Multiple phases. Small dimensional scales <1 µm

Microemulsion Phase Diagram 56

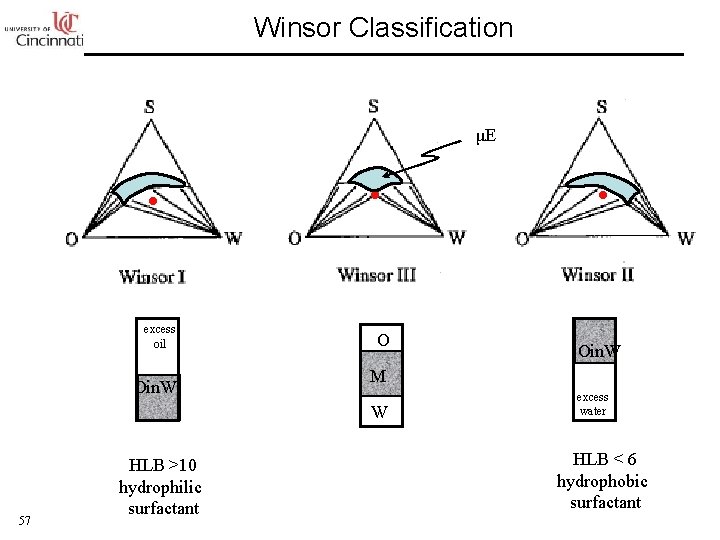
Winsor Classification μE excess oil Oin. W O M W 57 HLB >10 hydrophilic surfactant Oin. W excess water HLB < 6 hydrophobic surfactant

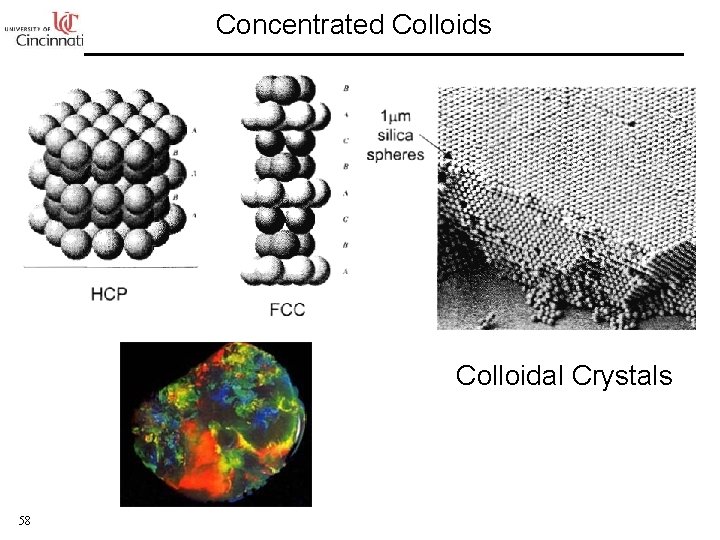
Concentrated Colloids Colloidal Crystals 58

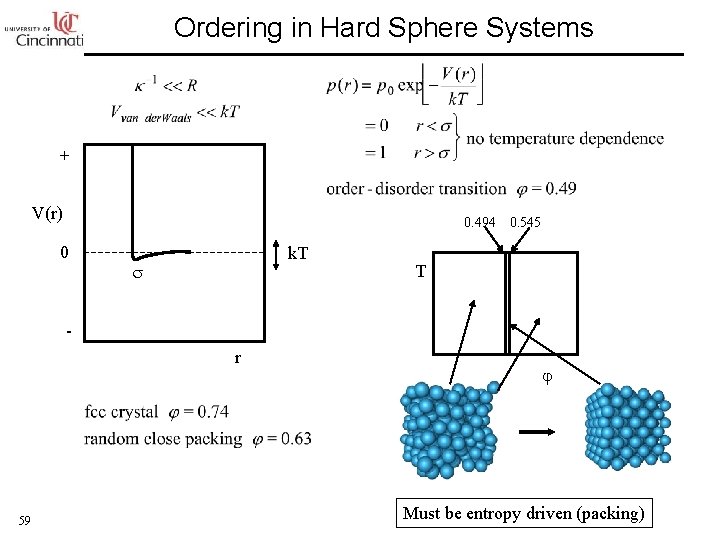
Ordering in Hard Sphere Systems + V(r) 0. 494 0 k. T 0. 545 T r 59 Must be entropy driven (packing)

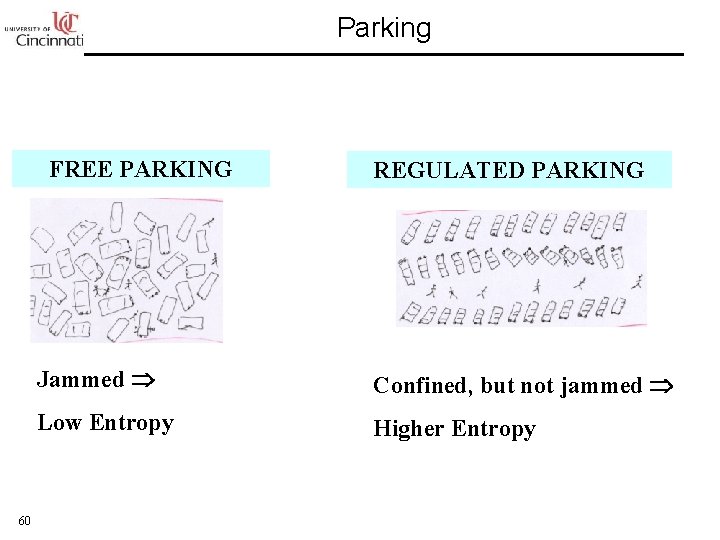
Parking FREE PARKING 60 REGULATED PARKING Jammed Confined, but not jammed Low Entropy Higher Entropy

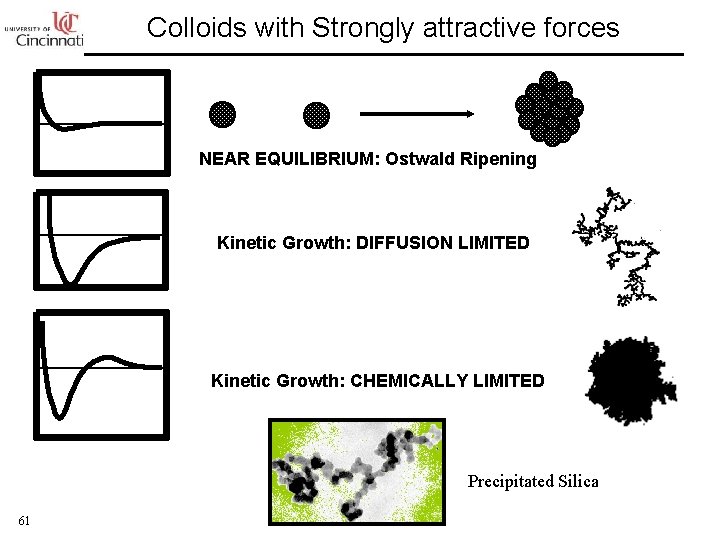
Colloids with Strongly attractive forces NEAR EQUILIBRIUM: Ostwald Ripening Kinetic Growth: DIFFUSION LIMITED Kinetic Growth: CHEMICALLY LIMITED Precipitated Silica 61

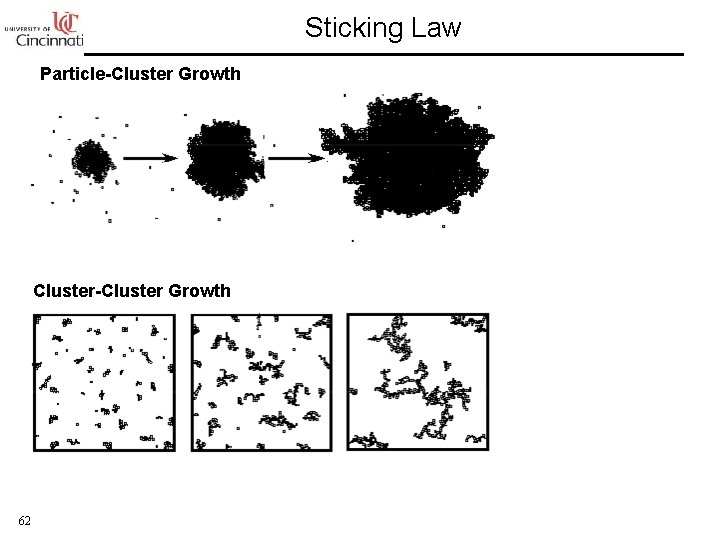
Sticking Law Particle-Cluster Growth Cluster-Cluster Growth 62

Transport Diffusion-Limited Ballistic Reaction-Limited (Independent of transport) 63

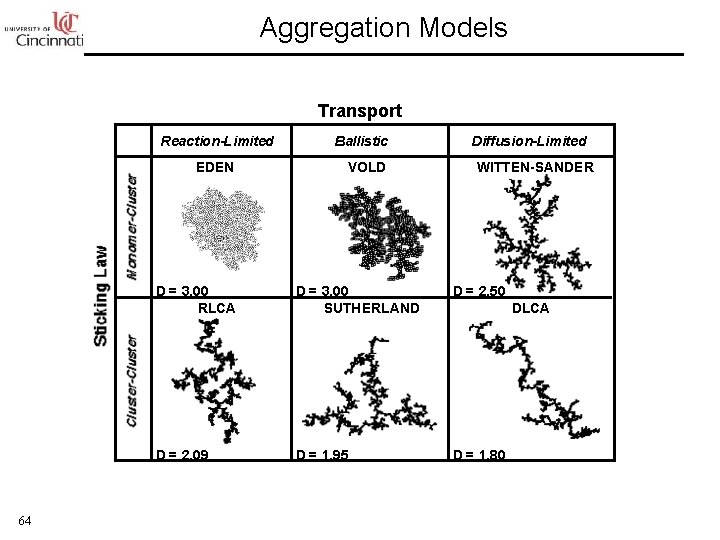
Aggregation Models Transport Reaction-Limited EDEN 64 Ballistic VOLD Diffusion-Limited WITTEN-SANDER D = 3. 00 RLCA D = 3. 00 SUTHERLAND D = 2. 50 D = 2. 09 D = 1. 95 D = 1. 80 DLCA

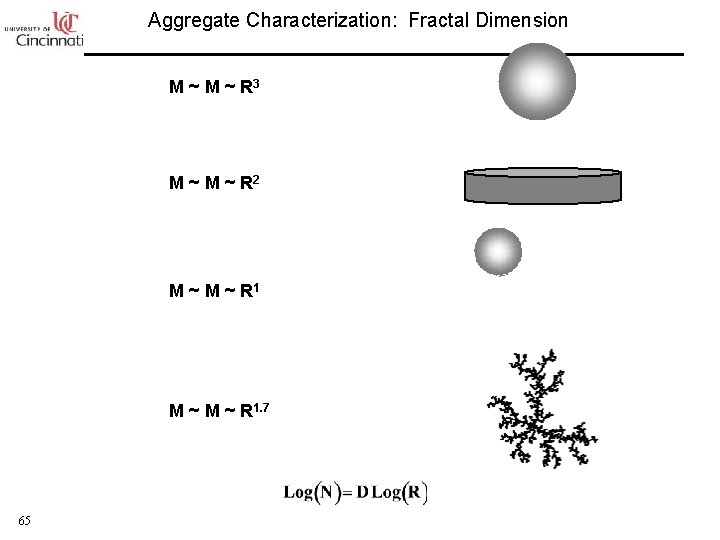
Aggregate Characterization: Fractal Dimension M ~ R 3 M ~ R 2 M ~ M ~ R 1. 7 65

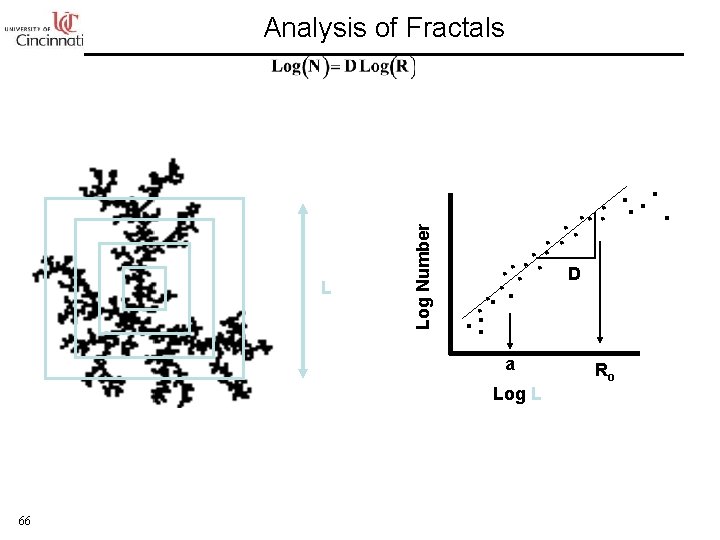
L Log Number Analysis of Fractals D a Log L 66 Ro

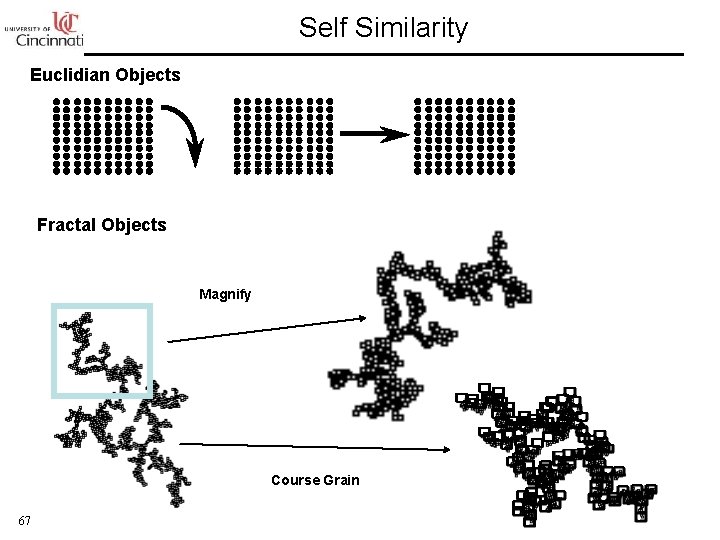
Self Similarity Euclidian Objects Fractal Objects Magnify Course Grain 67

68

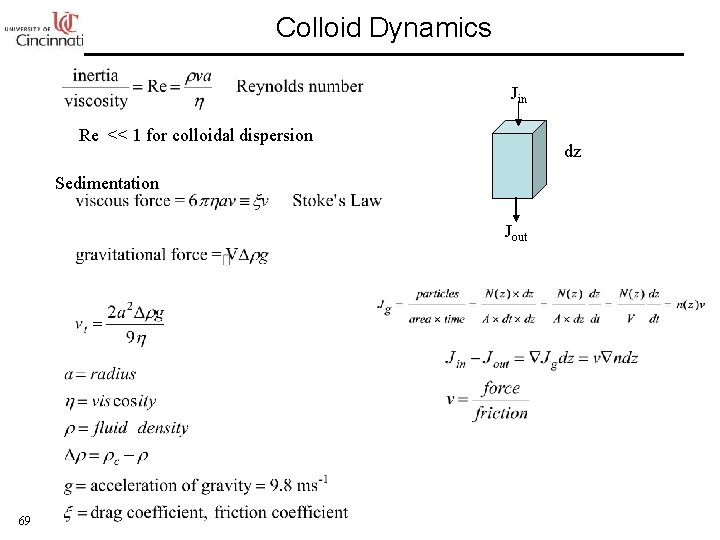
Colloid Dynamics Jin Re << 1 for colloidal dispersion dz Sedimentation Jout 69

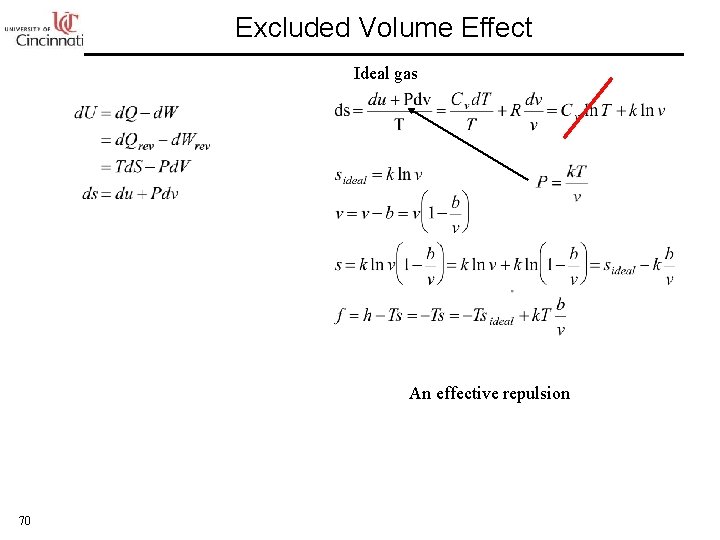
Excluded Volume Effect Ideal gas An effective repulsion 70

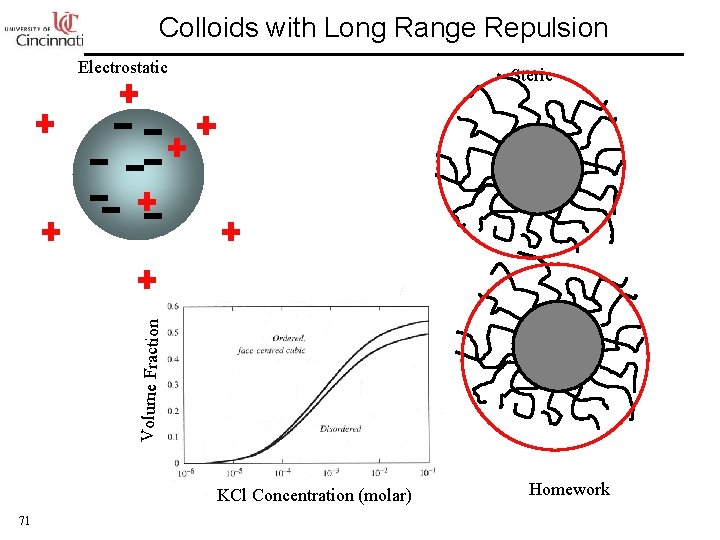
Colloids with Long Range Repulsion Electrostatic Volume Fraction Steric KCl Concentration (molar) 71 Homework

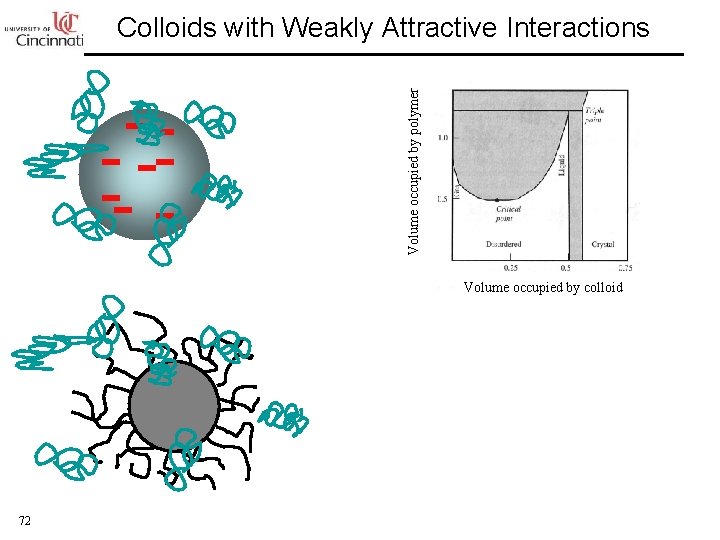
Volume occupied by polymer Colloids with Weakly Attractive Interactions Volume occupied by colloid 72

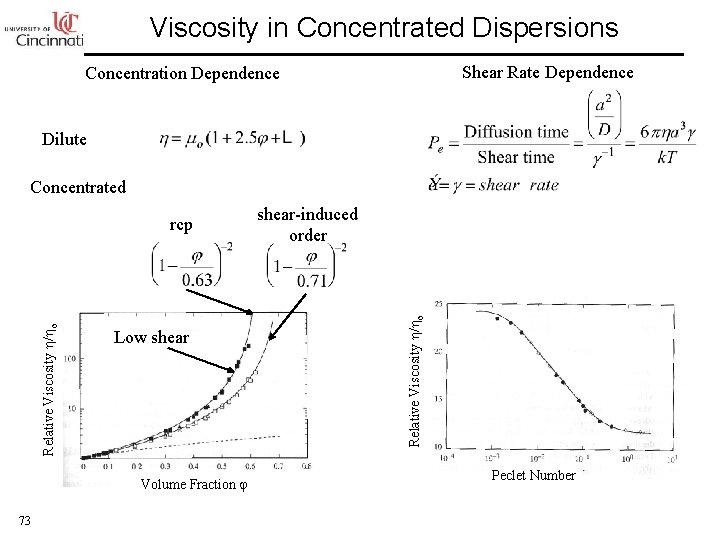
Viscosity in Concentrated Dispersions Shear Rate Dependence Concentration Dependence Dilute Concentrated Low shear Volume Fraction 73 shear-induced order Relative Viscosity / o rcp Peclet Number

74

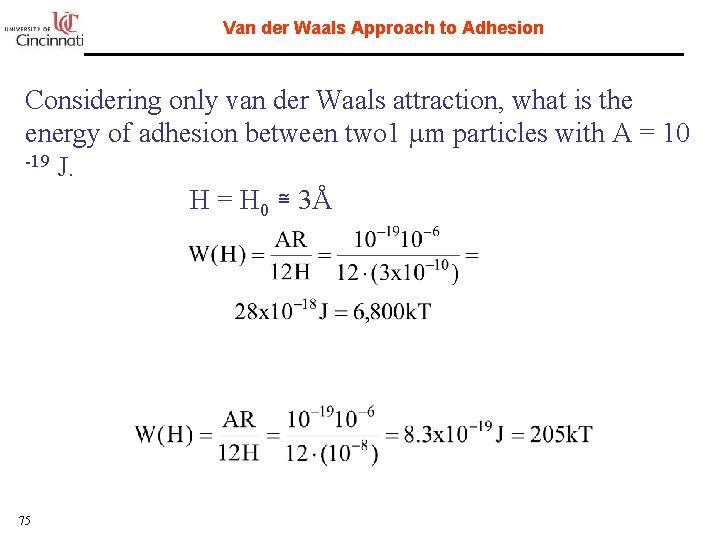
Van der Waals Approach to Adhesion Considering only van der Waals attraction, what is the energy of adhesion between two 1 mm particles with A = 10 -19 J. H = H 0 ≅ 3Å What if the particles were separated by 10 nm 75

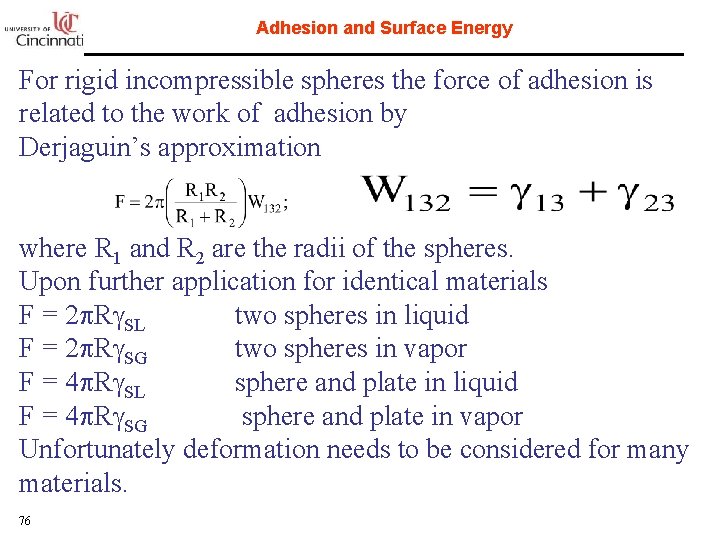
Adhesion and Surface Energy For rigid incompressible spheres the force of adhesion is related to the work of adhesion by Derjaguin’s approximation where R 1 and R 2 are the radii of the spheres. Upon further application for identical materials F = 2 p. Rg. SL two spheres in liquid F = 2 p. Rg. SG two spheres in vapor F = 4 p. Rg. SL sphere and plate in liquid F = 4 p. Rg. SG sphere and plate in vapor Unfortunately deformation needs to be considered for many materials. 76

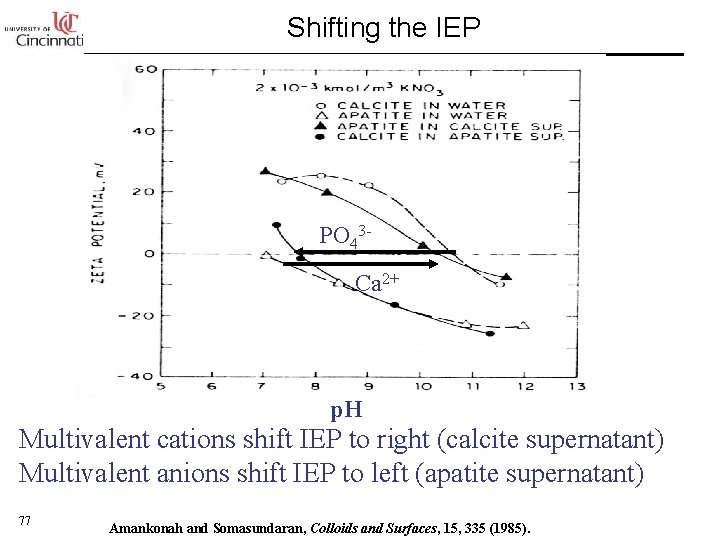
Shifting the IEP PO 43 Ca 2+ p. H Multivalent cations shift IEP to right (calcite supernatant) Multivalent anions shift IEP to left (apatite supernatant) 77 Amankonah and Somasundaran, Colloids and Surfaces, 15, 335 (1985).

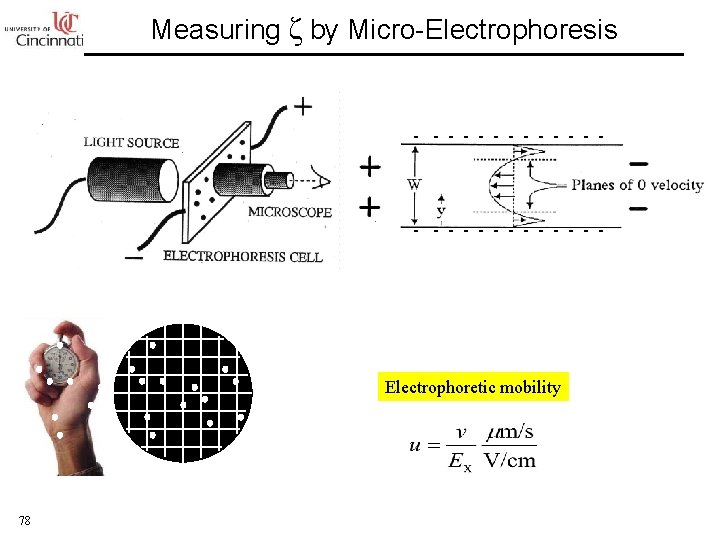
Measuring ζ by Micro-Electrophoresis - - - - - - - Electrophoretic mobility 78

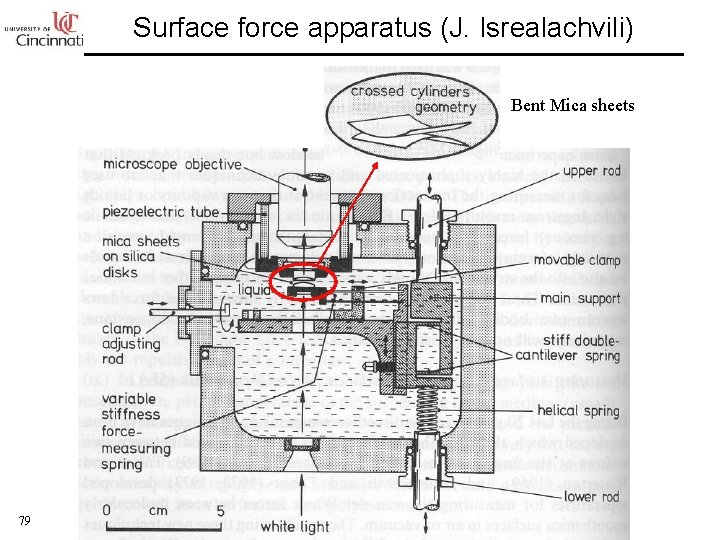
Surface force apparatus (J. Isrealachvili) Bent Mica sheets 79

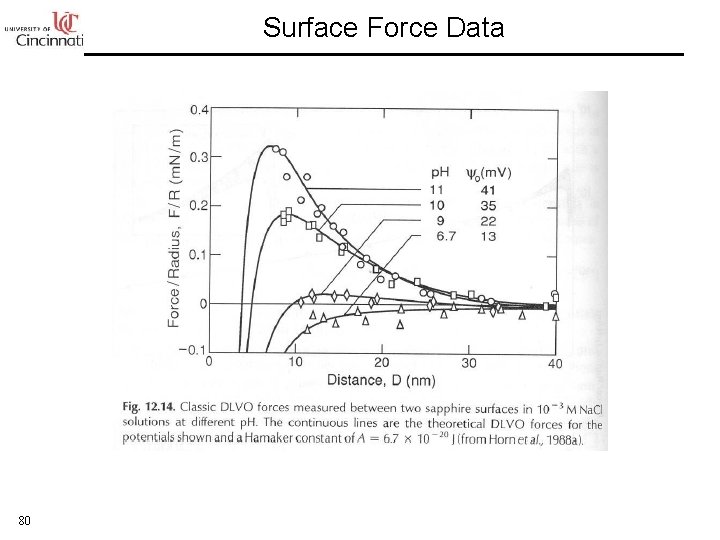
Surface Force Data 80

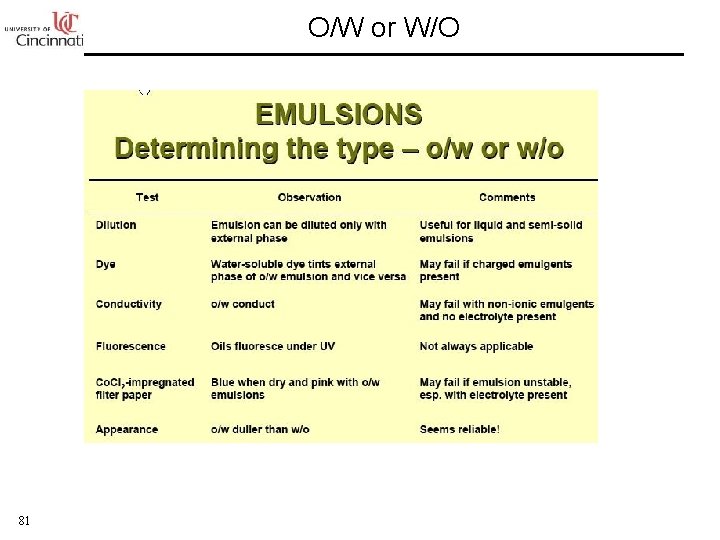
O/W or W/O 81

82

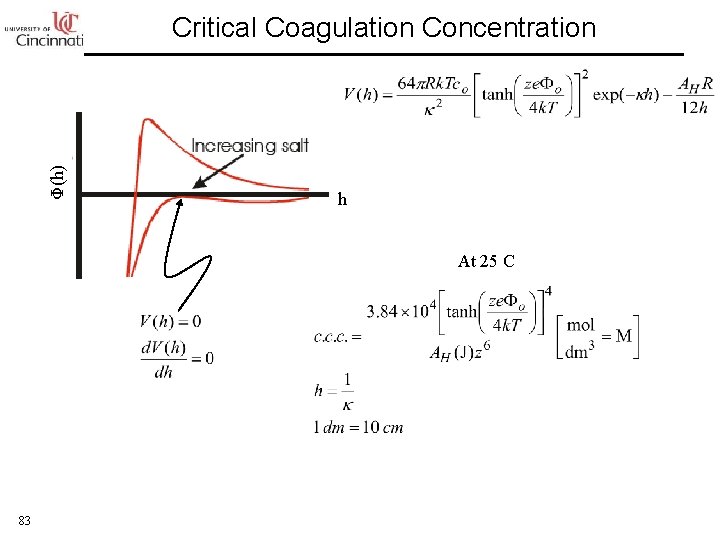
Φ(h) Critical Coagulation Concentration h At 25 C 83

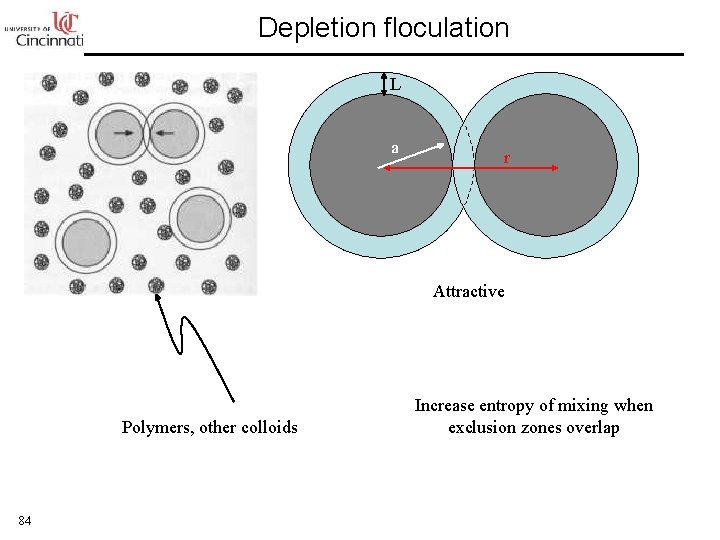
Depletion floculation L a r Attractive Polymers, other colloids 84 Increase entropy of mixing when exclusion zones overlap

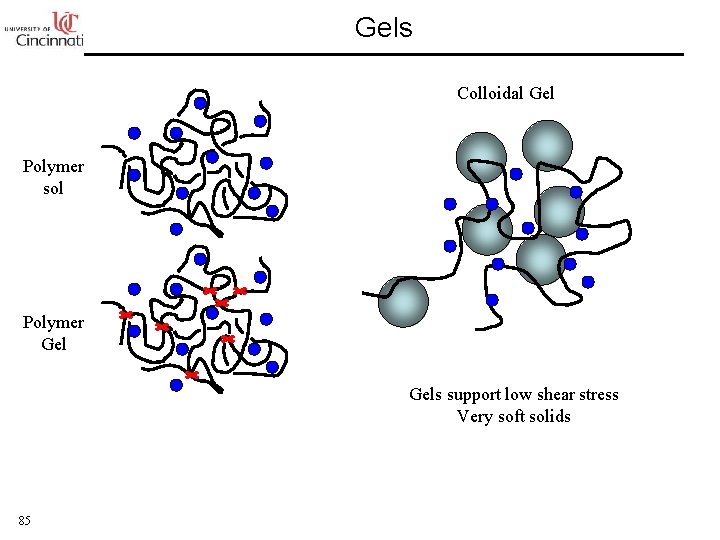
Gels Colloidal Gel Polymer sol Polymer Gels support low shear stress Very soft solids 85

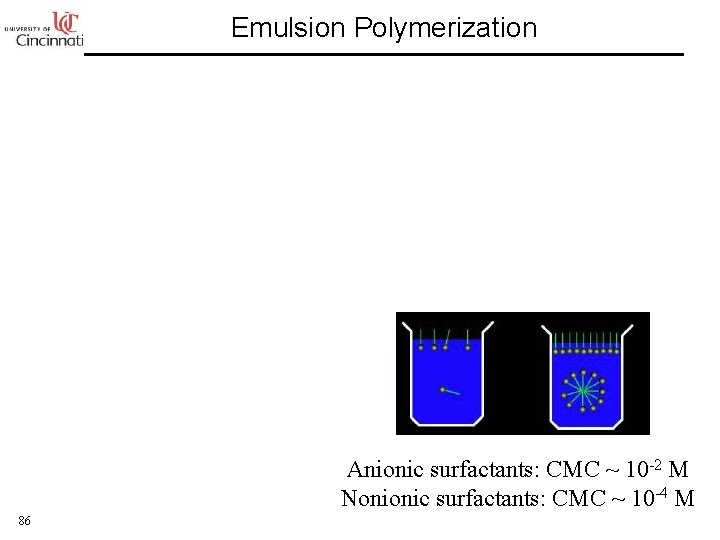
Emulsion Polymerization Anionic surfactants: CMC ~ 10 -2 M Nonionic surfactants: CMC ~ 10 -4 M 86

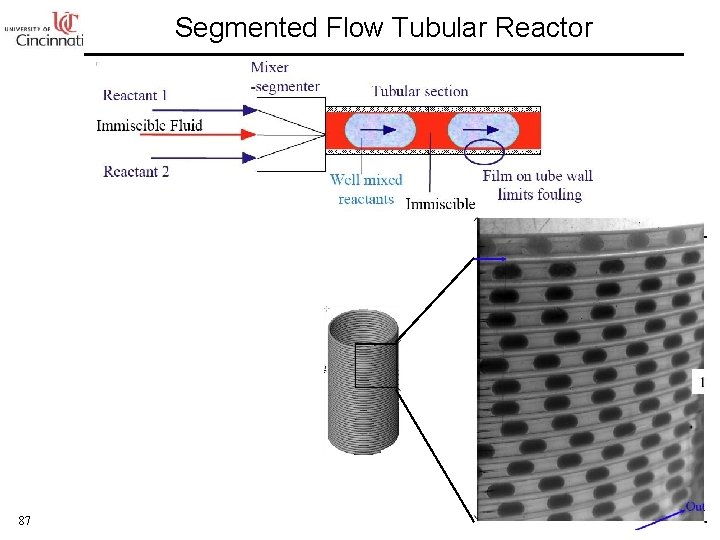
Segmented Flow Tubular Reactor 87