CHAPTER 2 Activity 3 Solutions Suspensions Colloids WHAT













- Slides: 25

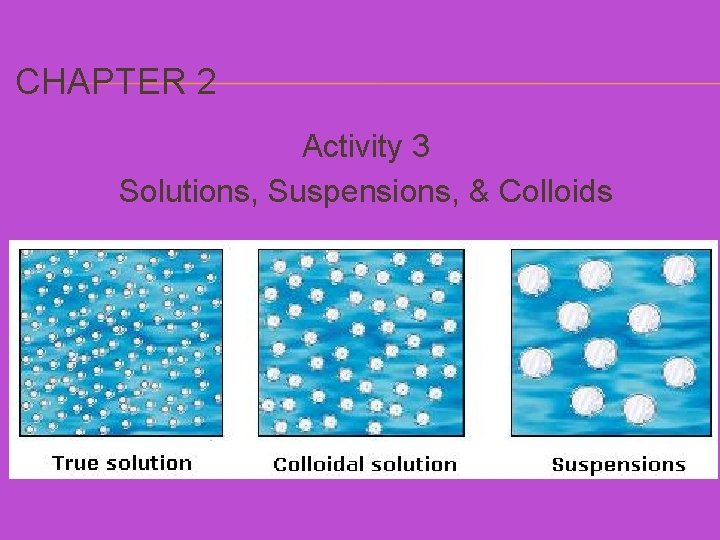
CHAPTER 2 Activity 3 Solutions, Suspensions, & Colloids


WHAT DO YOU THINK? ? ? ✕ One way to get different types of materials is to just mix them together. Lots of different things can happen when materials are mixed. You can get some good food or you can get dynamite. Each kind of mixture has its own characteristics. Taking mixtures apart is a different story. ✕ Is it easier to separate milk from coffee or milk from a bowl of cereal? Why? ?

WHICH ONE IS EASIER? ✕ Milk + and cereal- You can use a colander. ✕ Milk and coffee- You would have to boil it (distill it)

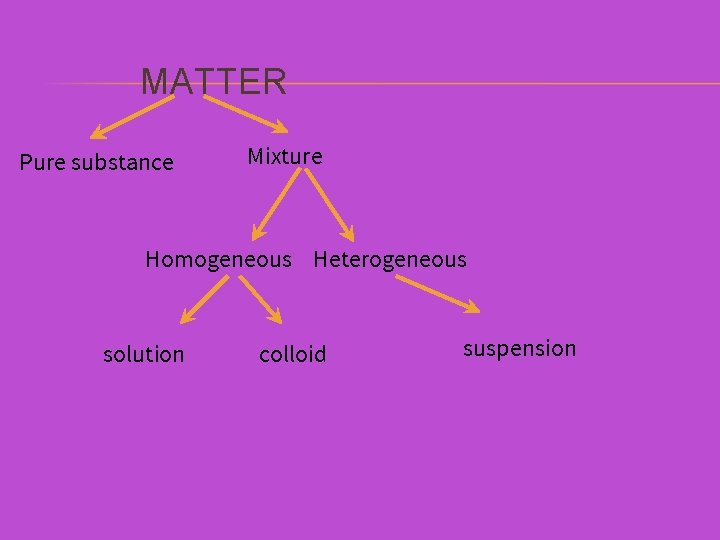
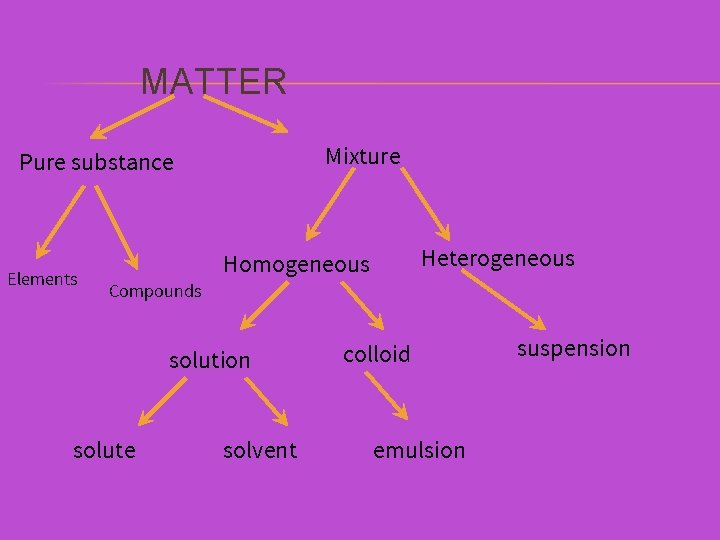
MATTER Pure substance Mixture

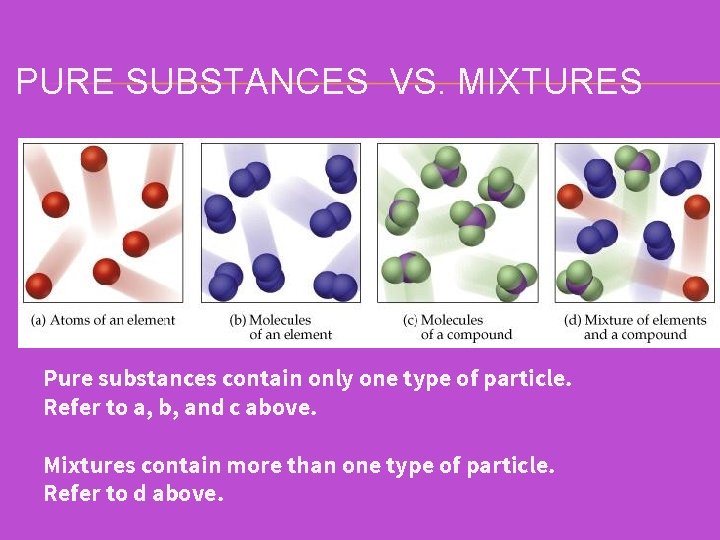
PURE SUBSTANCES VS. MIXTURES Pure substances contain only one type of particle. Refer to a, b, and c above. Mixtures contain more than one type of particle. Refer to d above.

MATTER Pure substance Homogeneous Mixture Heterogeneous

MIXTURES Homogeneous or heterogeneous? ? ?

✕ In a HOMOGENEOUS MIXTURE, the different components are not individually visible. Can be classified as a solution or a colloid

✕ In a HETEROGENEOUS MIXTURE, the different components are individually visible and will tend to separate over time. Referred to as a suspension

MATTER Pure substance Mixture Homogeneous Heterogeneous solution colloid suspension

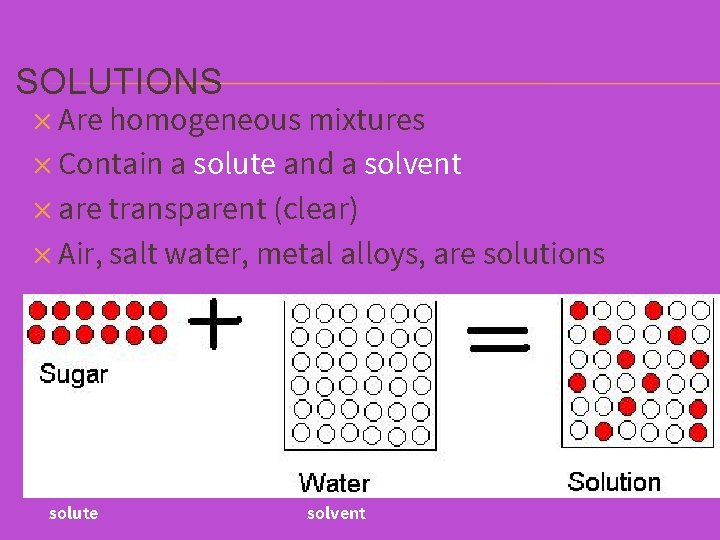
SOLUTIONS ✕ Are homogeneous mixtures ✕ Contain a solute and a solvent ✕ are transparent (clear) ✕ Air, salt water, metal alloys, are solutions solute solvent

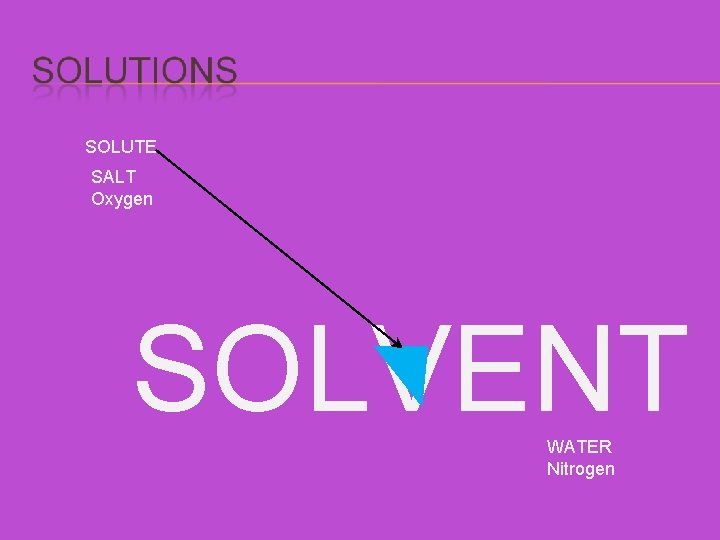
SOLUTE SALT Oxygen SOLVENT WATER Nitrogen

SUGAR GLASS ✕ Sugar glass is a fake glass made from kitchen ingredients. It's used in film stunts because the edges aren't as sharp and dangerous. ✕ It is a solution of water, light corn syrup, sugar and cream of tartar.

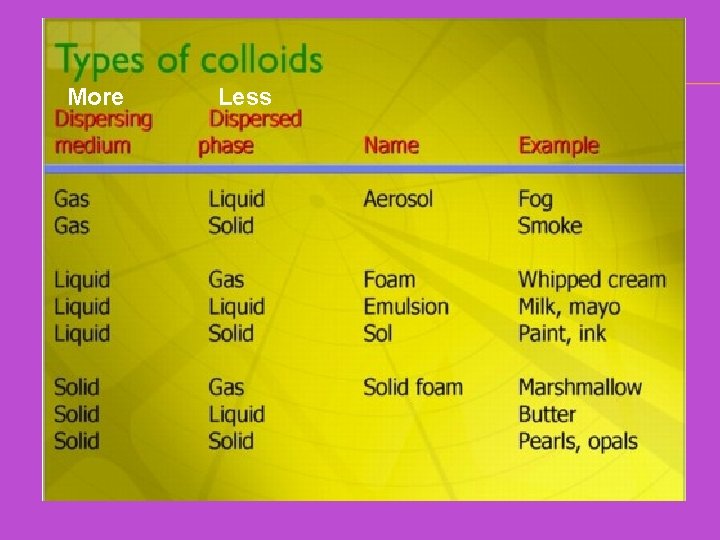
COLLOIDS ✕ Homogeneous ✕ Solute particles are larger than those in a solution ✕ Most commonly transparent ✕ A liquid-liquid colloid is an emulsion ✕ Difficult to distinguish from a solution with the naked eye. So how can you tell solutions and colloids apart?

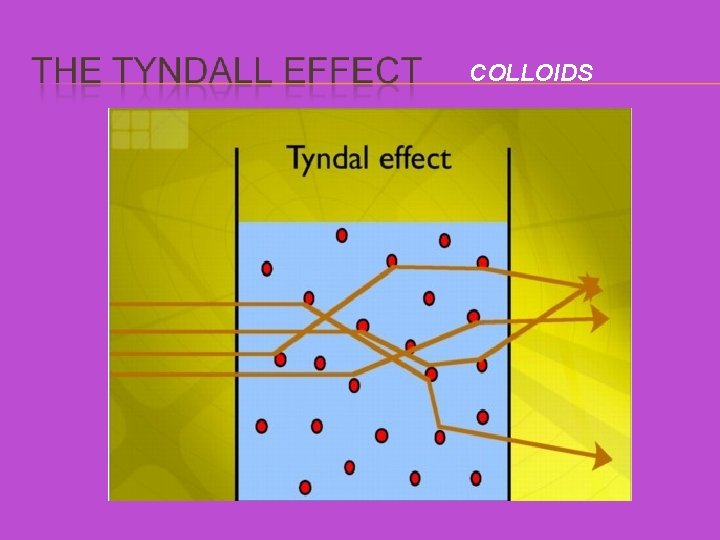
THE TYNDALL EFFECT When a beam of light is shone through a colloid, the larger solute particles scatter the beam of light, enabling you to see the beam as it goes through the mixture. So how can you tell solutions and colloids apart? Solutions don’t scatter light, so you won’t see the beam going through the solution

FOG IS A COLLOID

COLLOIDS

COLLOID SOLUTION

More Less

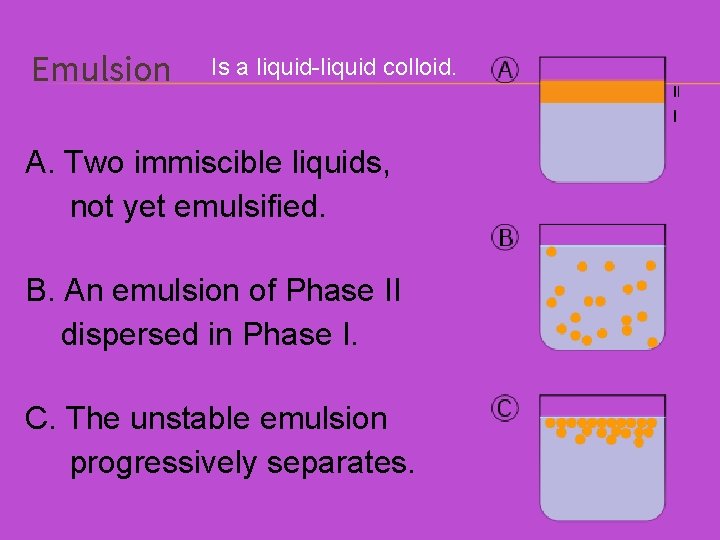
EMULSION Is a liquid-liquid colloid. Milk is a natural emulsion of liquid fat in a watery liquid

Emulsion Is a liquid-liquid colloid. A. Two immiscible liquids, not yet emulsified. B. An emulsion of Phase II dispersed in Phase I. C. The unstable emulsion progressively separates.

SUSPENSIONS ✕ Heterogeneous mixture with very large solute particles ✕ Particles can be seen with the naked eye ✕ Particles will settle out in time (OJ, Aerosol Cans) ✕ The particles can be separated with a filter

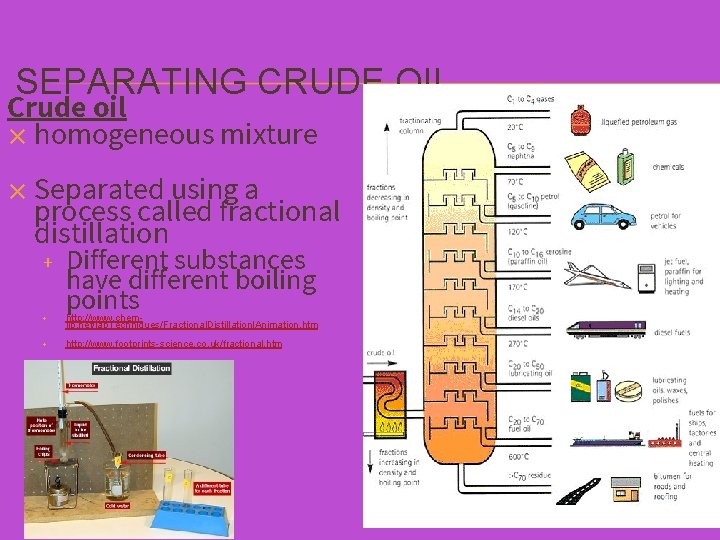
SEPARATING CRUDE OIL Crude oil ✕ homogeneous mixture ✕ Separated using a process called fractional distillation + + + Different substances have different boiling points http: //www. chemilp. net/lab. Techniques/Fractional. Distillationl. Animation. htm http: //www. footprints-science. co. uk/fractional. htm

MATTER Mixture Pure substance Elements Heterogeneous Homogeneous Compounds solution solute solvent colloid emulsion suspension