Three Phases of Matter Classification of Matter Solutions

















- Slides: 27

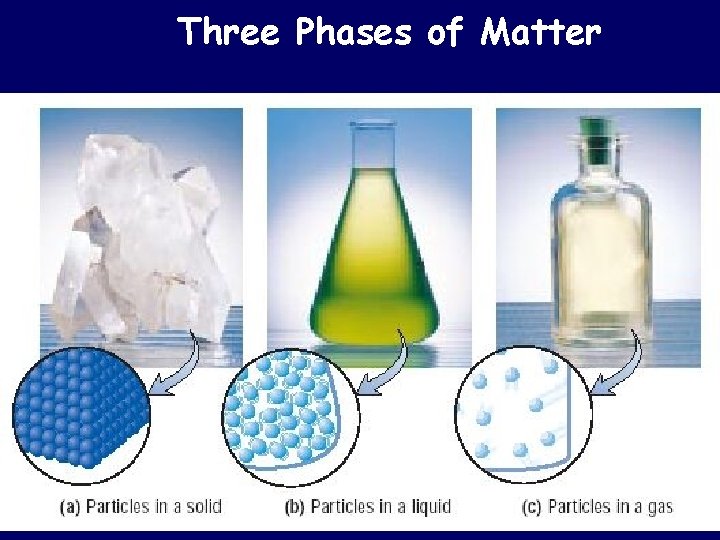
Three Phases of Matter

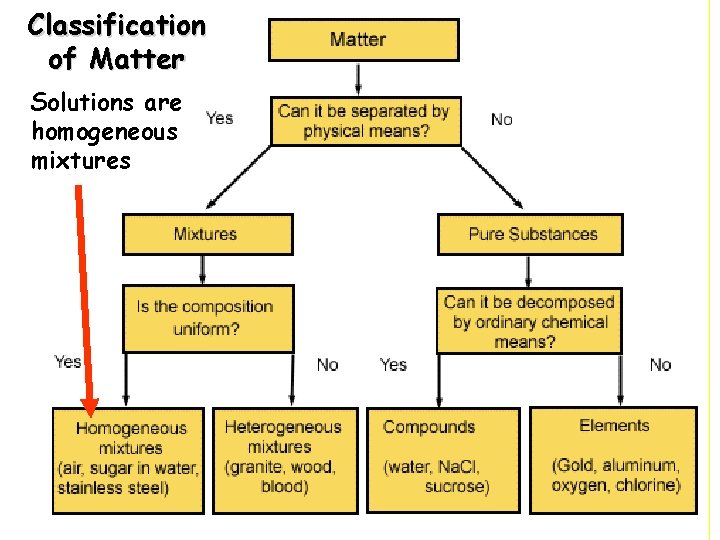
Classification of Matter Solutions are homogeneous mixtures

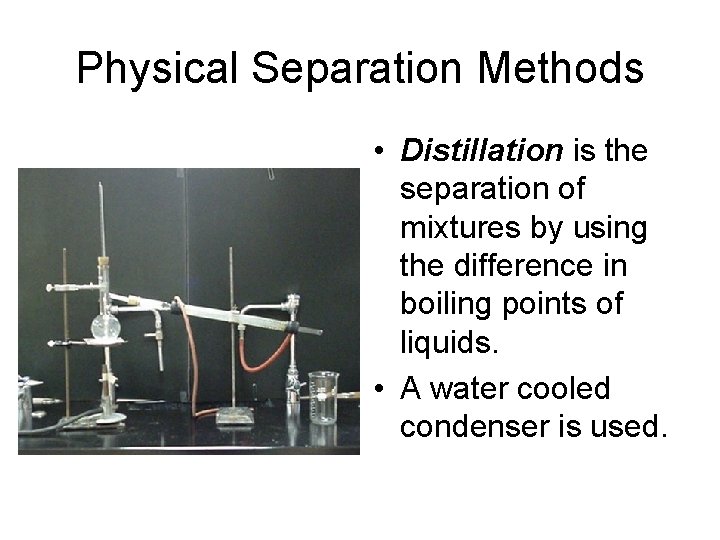
Physical Separation Methods • Distillation is the separation of mixtures by using the difference in boiling points of liquids. • A water cooled condenser is used.

Elemental Samples • Zinc, copper, lead, carbon, sulfur

Colored Compounds • Cobalt(II) chloride, Iron(II) sulfate, Potassium dichromate, Potassium chromate, Nickel(II) nitrate, copper(II) sulfate

Impure Matter - Mixture • “A mixture is a blend of two or more kinds of matter, each of which retains its own identity and properties. ” • A mixture is made up of two or more substances that are not chemically combined.

Mixtures • Mixtures can be separated by simple physical means. • Two mixtures containing the same substances may not have the same proportions. • Example: Very salty water versus barely salty water. Very sweet sugar water versus slightly sweet sugar water.

Water and Dye Mixture • Two mixtures of the same substances may have different proportions.

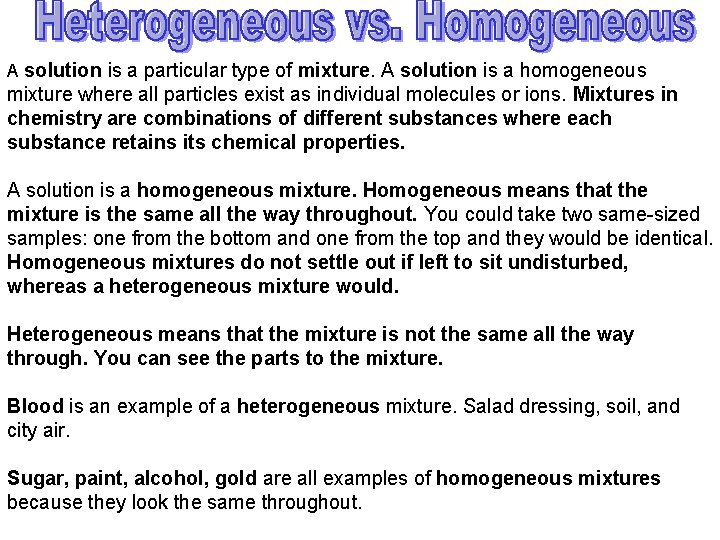
A solution is a particular type of mixture. A solution is a homogeneous mixture where all particles exist as individual molecules or ions. Mixtures in chemistry are combinations of different substances where each substance retains its chemical properties. A solution is a homogeneous mixture. Homogeneous means that the mixture is the same all the way throughout. You could take two same-sized samples: one from the bottom and one from the top and they would be identical. Homogeneous mixtures do not settle out if left to sit undisturbed, whereas a heterogeneous mixture would. Heterogeneous means that the mixture is not the same all the way through. You can see the parts to the mixture. Blood is an example of a heterogeneous mixture. Salad dressing, soil, and city air. Sugar, paint, alcohol, gold are all examples of homogeneous mixtures because they look the same throughout.

Homogeneous Mixture • Homogeneous mixtures are uniform in composition. • They have the same proportion of components throughout. • Homogeneous mixtures are called solutions. • A solution is a particular type of mixture. A solution is a homogeneous mixture where all particles exist as individual molecules or ions. • Salt water, kool aid, & gatorade all solutions

Heterogeneous Mixture • Heterogeneous mixtures are not uniform throughout. • Clay particles in water are an example.

Heterogeneous Mixtures • Sand water on the left and sand gravel on the right.

Solution = Solute + Solvent Solute A solute is the dissolved substance in a solution. What is getting dissolved. Salt in salt water Sugar in soda drinks Carbon dioxide in soda drinks Solvent A solvent is the dissolving medium in a solution. What is doing the dissolving. Water in salt water Water in soda

Particle Size • The particle size for homogeneous and heterogeneous mixtures is defined. – The solute particles for homogeneous mixtures are so small that they cannot be seen. – Heterogeneous mixture particles are visible.

Suspensions and Colloids Suspensions and colloids are NOT solutions. Suspensions: The particles are so large that they settle out of the solvent if not constantly stirred. Colloids: The particles intermediate in size between those of a suspension and those of a solution.

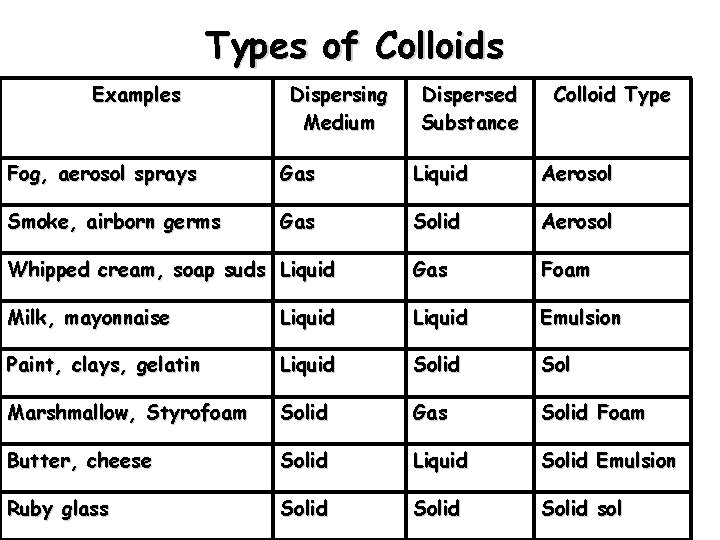
Types of Colloids Examples Dispersing Medium Dispersed Substance Colloid Type Fog, aerosol sprays Gas Liquid Aerosol Smoke, airborn germs Gas Solid Aerosol Whipped cream, soap suds Liquid Gas Foam Milk, mayonnaise Liquid Emulsion Paint, clays, gelatin Liquid Sol Marshmallow, Styrofoam Solid Gas Solid Foam Butter, cheese Solid Liquid Solid Emulsion Ruby glass Solid sol

Colloidal Suspension • Fog

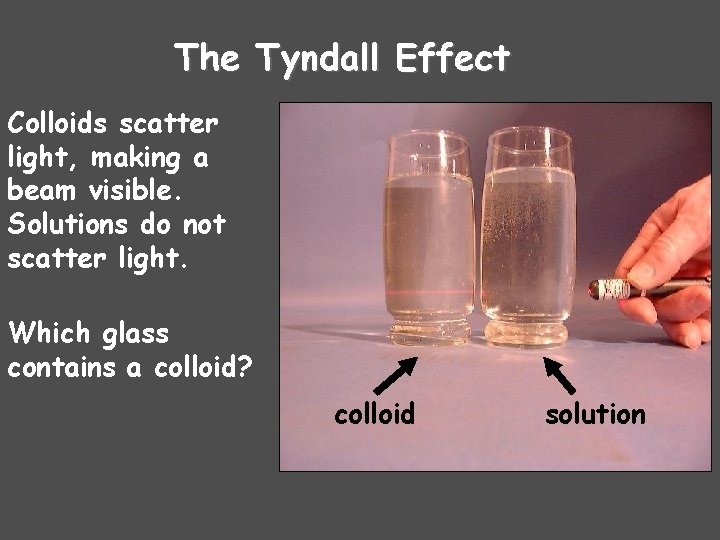
The Tyndall Effect Colloids scatter light, making a beam visible. Solutions do not scatter light. Which glass contains a colloid? colloid solution

A physical property of a substance is anything that can be observed without changing the identity of the substance. The observations usually consist of some type of numerical measurement, although sometimes there is a more qualitative (non-numerical) description of the property. Here are some of the more common ones: melting point, electrical conductivity, color, density, boiling point, thermal conductivity, odor, hardness, solubility, magnetism, taste, volume, length, texture.

Groups of similar elements or compounds can be characterized by commonality in their physical properties. Metals have a whole bunch of physical properties that are similar. For example, metals are very ductile and very malleable. All easily conduct electricity and heat and all have a bright luster. These all reflect a commonality of structure.

A chemical property describes the way a substance may change or react to form other substances. If you see the word “REACTS” or “REACTIVITY that is a chemical property. There really isn't a set of chemical properties in the same way there is a set of physical properties. That's because the chemical properties are tied to the change. Here are some common chemical properties: (1) iron rusting. When iron (an element, symbol = Fe) rusts, it combines in a complex fashion with oxygen to form a reddish-colored compound called ferric oxide (formula = Fe 2 O 3). Not all substances rust. (2) glucose, mixed with yeast, ferments to make alcohol. Glucose (C 6 H 12 O 6) is a chemical compound which enzymes in yeast can use to make ethyl alcohol (C 2 H 5 OH). Not all substances ferment. (3) trinitrotoluene (TNT) reacts very, very fast when it is ignited. Among other products, it makes LOTS of nitrogen gas and LOTS of heat. Inside the proper container, it can cause an explosion. Not all substances can make an explosion. (4) flammability (combustion)- the ability of a substance to burn in the presence of oxygen. Some substances (wood, alcohol) are very flammable, others are not.

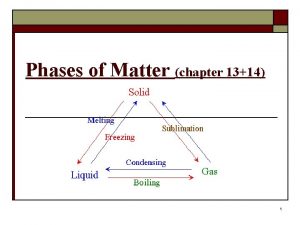
A physical change is any change NOT involving a change in the substance's chemical identity. Here are some examples: (1) any phase change. Moving between solid, liquid and gas involves only the amount of energy in the sample (this amount is the subject of future lessons). There is no effect on the chemical identity of the substance. For example, water remains water, no matter if it solid, liquid or gas. (2) grinding something into a powder or dissolving. Or the reverse process of making a bigger lump of stuff, say by melting lots of small pellets of copper into one big piece. (3) iron (and other metals) can be made to be magnetic. This change in no way affects the chemical identity of the element. Iron that is magnetized rusts just as easily as iron that is not magnetized.

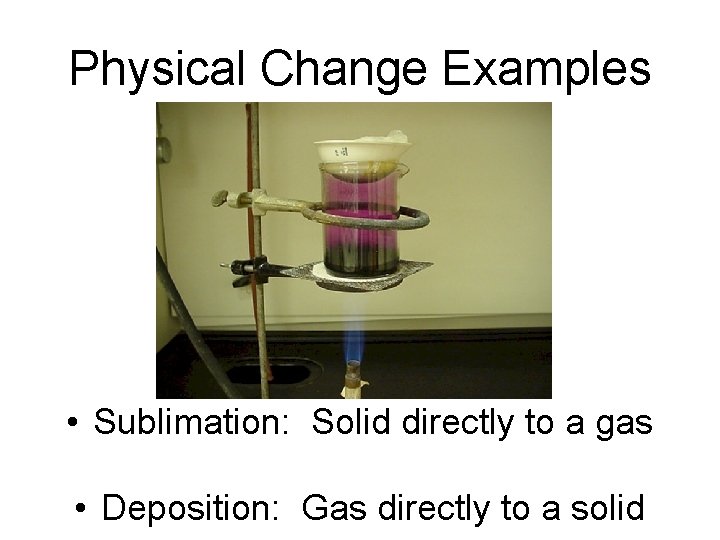
Physical Change Examples • Sublimation: Solid directly to a gas • Deposition: Gas directly to a solid

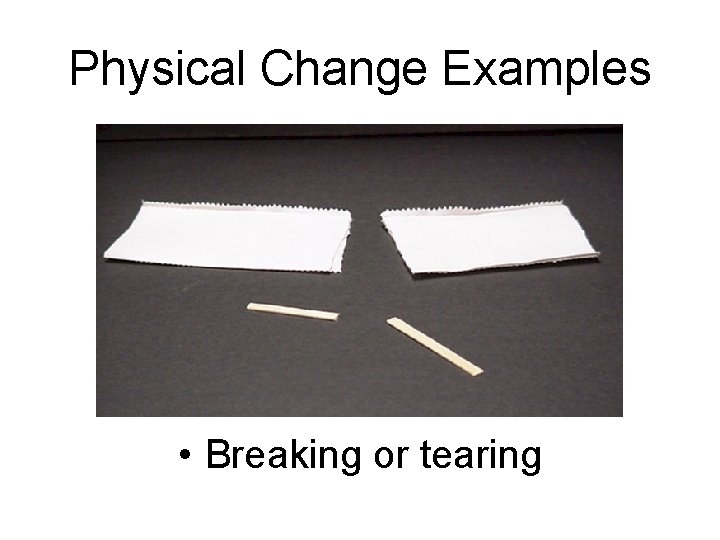
Physical Change Examples • Breaking or tearing

Physical Change Examples • Freezing or melting

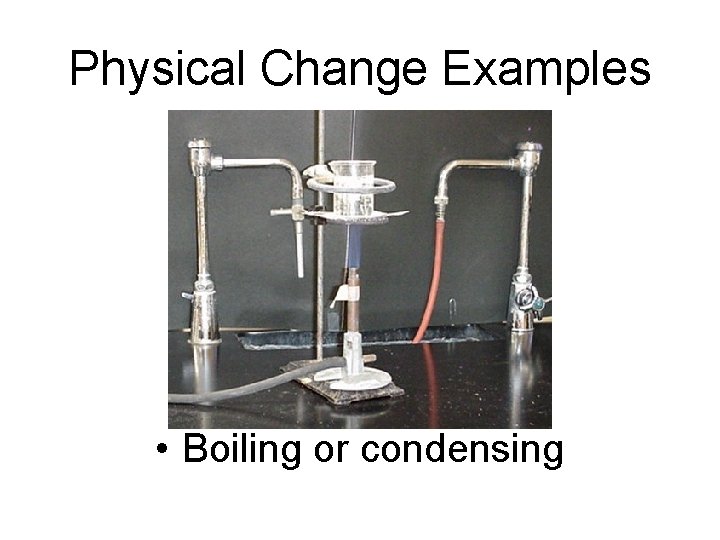
Physical Change Examples • Boiling or condensing

A chemical change means that the reacting substances are changed into new substances. The actual atoms involved remain, they are simply rearranged. The rearrangement is called a chemical reaction. For example: 2 H 2 O ---> 2 H 2 + O 2 is a chemical reaction in which water is broken down into the hydrogen and oxygen which make it up. Notice how the amounts of hydrogen atoms (four) and oxygen atoms (two) do not change from one side of the arrow to the other. However, the arrangements of the atoms is different. Some chemical bonds (the one involved in the water) have been broken and some new chemical bonds (the one in hydrogen and oxygen) have been formed.
 Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Four states of matter
Four states of matter Four states of matter
Four states of matter Phases changes of matter
Phases changes of matter 3 phases of matter
3 phases of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases States of matter foldable
States of matter foldable Phases of matter
Phases of matter Classification of matter concept map
Classification of matter concept map Three phases of action potential
Three phases of action potential Research proses
Research proses Perioperative vs intraoperative
Perioperative vs intraoperative Importance of evaluating channel member performance
Importance of evaluating channel member performance Three phases of film production
Three phases of film production Theme of dulce et decorum est
Theme of dulce et decorum est What are the three stages of writing?
What are the three stages of writing? 3 phases of muscle reading
3 phases of muscle reading The three phases of diesel ignition include
The three phases of diesel ignition include Three phases of database design
Three phases of database design Osha 1910 120
Osha 1910 120 Eutectic mixture
Eutectic mixture Pattinson process phase diagram
Pattinson process phase diagram Define degree of freedom
Define degree of freedom Gray matter
Gray matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Median and lateral apertures
Median and lateral apertures