PHASES OF MATTER PHASES SOLID Particles relatively close











- Slides: 32

PHASES OF MATTER

PHASES ___________

SOLID Particles relatively close together Retains _________ Relatively _____ densities Hard to ______ Does not flow easily Types Crystalline- regular order/ pattern to particles Amorphous- no regular pattern to particles

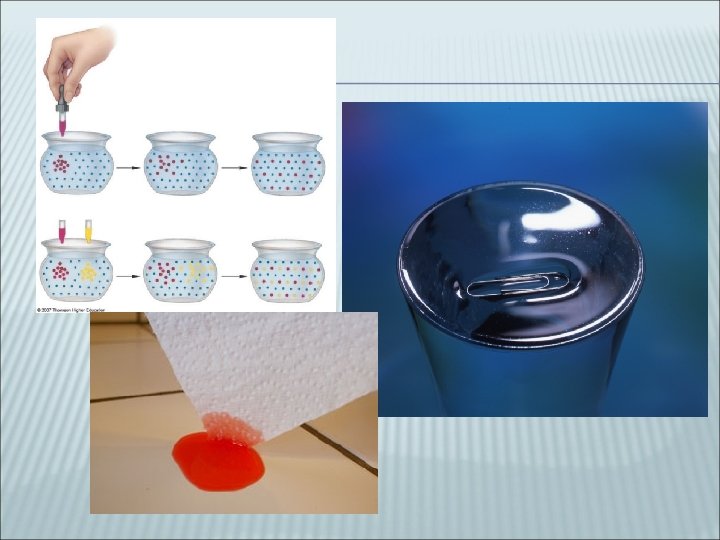
LIQUID Particles more spaced apart Retains ________________ Intermediate densities Hard to compress Flows easily (______) Can ____- liquid molecules spread out through another liquid Can display _______- attraction of molecules at surface to each other Can display ________- attraction of liquid to solid surface causing it to flow


GAS Particles spaced very far apart Does ____ retain volume or shape Very low densities Compressible Flows easily (fluidity) Can diffuse and go through _________ collisions (no loss of energy during collision) Ideal vs Real Gases Ideal- no attraction between particles Real- gases where particles are attracted to each other

PLASMA Occurs at very high ____________ Also a fluid Mix of neutral atoms, free electrons, and ions

DENSITY __________ property Mass per unit volume of substance (_______) Units Solids and Liquids _____________ Gases _________ Density controls placement of fluids and solids Less dense objects or fluids move to the top More dense objects or fluids move to the bottom

DENSITY (CONT) D = m/V Mass Measured on a _______ Volume Solid Regular Shape- can be _____ from other measurements Irregular Shape- can be found by ____________ Liquid Can and Gas be measured with instruments such as _____________

HEAT Amount of _______ transferred from one substance to another Represented by ____ with units in ______ When heat transfers, it affects the ________ of the substances

TEMPERATURE Measure of the ________ in a sample High temperatures mean the particles are moving _____ Theoretically if the particles weren’t moving at all, the temperature would be 0 Kelvin (________) Remember K = °C + 273. 15

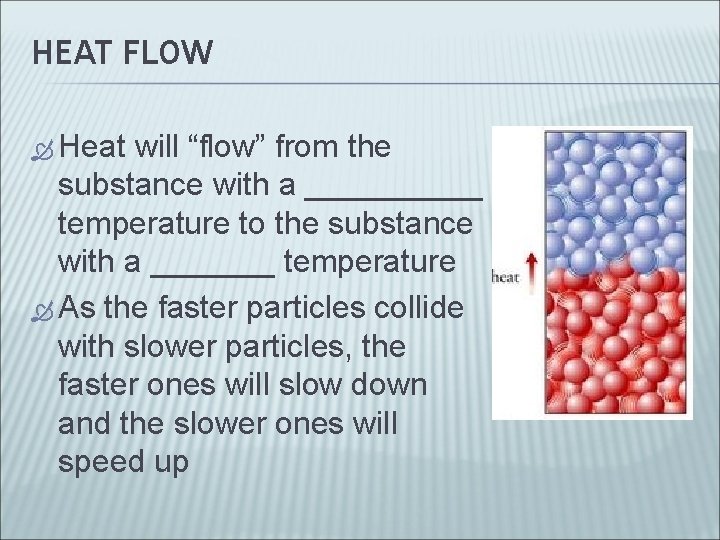
HEAT FLOW Heat will “flow” from the substance with a _____ temperature to the substance with a _______ temperature As the faster particles collide with slower particles, the faster ones will slow down and the slower ones will speed up

HEAT AND TEMPERATURE CHANGE When heat transfers, it affects the temperatures of the substances involved in the transfer How much will the temperature change? Dependent on ______________________________

AMOUNT OF HEAT TRANSFERRED The more heat transferred, the greater the temperature change If heat is ______ by the sample q is ________ Final temperature will be higher than the initial temperature If heat is _____ by the sample q is _______ Final temperature will be lower than the initial temperature

MASS OF SAMPLE A heat transfer will cause a bigger temperature change to a smaller mass than it will to a larger mass.

COMPOSITION OF THE SAMPLE Different substances absorb/release heat in different ways. _________(c) – is the amount of heat needed to change 1 gram of a particular substance by 1 °C. Each type of substance has a different value

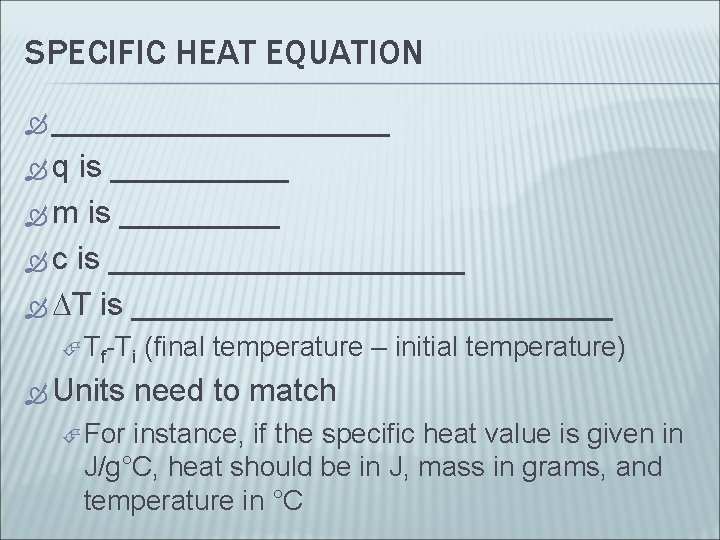
SPECIFIC HEAT EQUATION __________ q is _____ m is _____ c is __________ T is ______________ Tf-Ti Units For (final temperature – initial temperature) need to match instance, if the specific heat value is given in J/g°C, heat should be in J, mass in grams, and temperature in °C

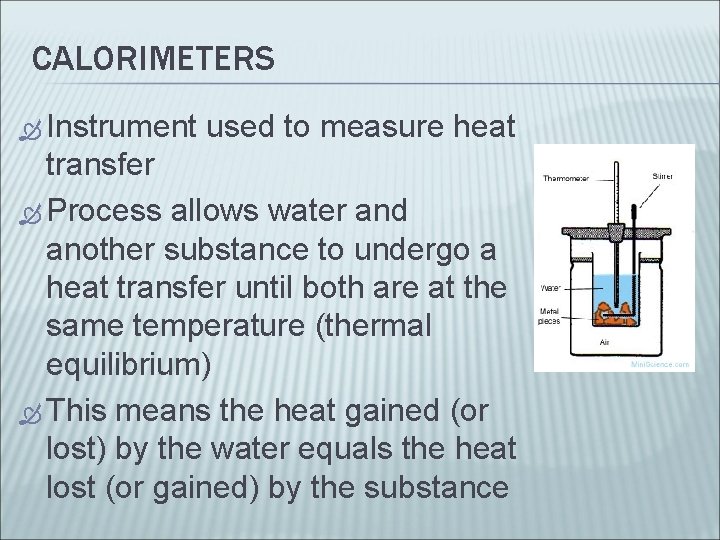
CALORIMETERS Instrument used to measure heat transfer Process allows water and another substance to undergo a heat transfer until both are at the same temperature (thermal equilibrium) This means the heat gained (or lost) by the water equals the heat lost (or gained) by the substance

ABSORBING HEAT Solid absorbs heat and temperature increases (molecules moving faster) Reaches a point that movement weakens IMF’s enough to allow flow (melting point) Heat is still absorbed but temperature does not increase Liquid absorbs heat and temperature increases (molecules moving faster) Reaches a point that movement weakens IMF’s enough that they essentially no longer exist (boiling point) Heat is still absorbed but temperature does not increase

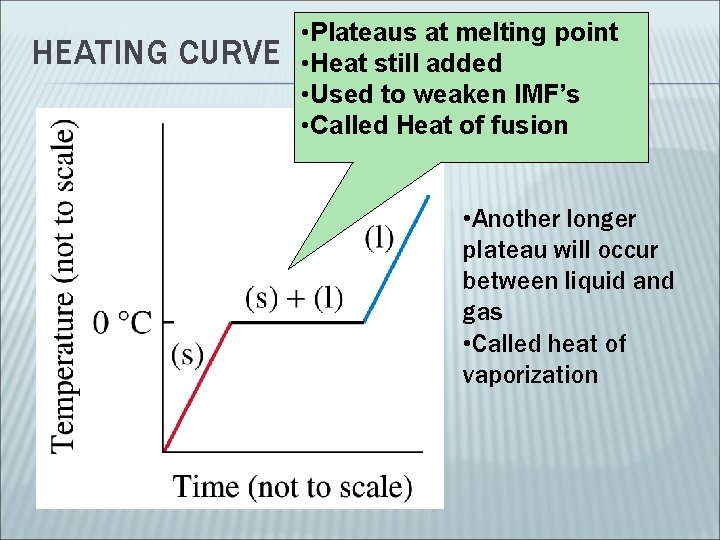
HEATING CURVE • Plateaus at melting point • Heat still added • Used to weaken IMF’s • Called Heat of fusion • Another longer plateau will occur between liquid and gas • Called heat of vaporization

RELEASING HEAT Heat is released from the gas and temperature decreases (molecules moving slower) Reaches a point that molecules are close enough for IMF’s to be reestablished (condensation point) Heat is still released but temperature does not decrease Heat is released from the liquid and temperature decreases (molecules moving slower) Reaches a point that molecules are close enough for IMF’s to strengthen (freezing point) Heat is still released but temperature does not decrease is removed from the solid

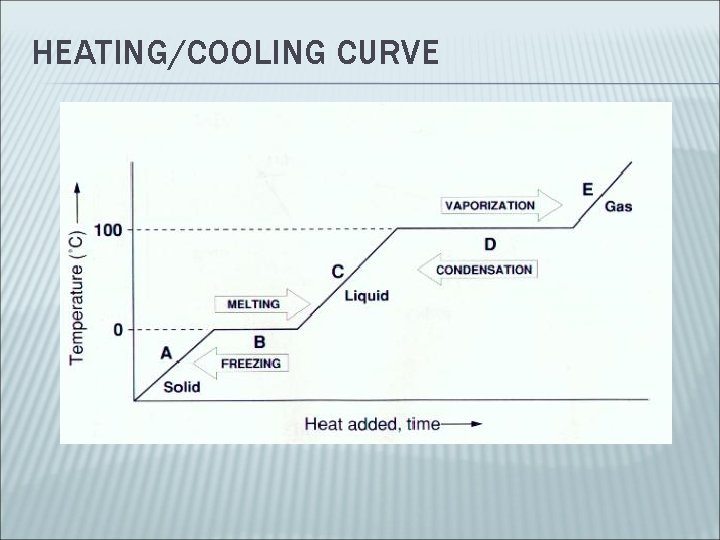
HEATING/COOLING CURVE

PHASE CHANGES Solid Liquid = ______ Liquid Solid = ______ Occurs at melting/ freezing point Solid Liquid Gas = ________________ Gas Liquid = ___________ Occurs at boiling/condensation point Liquid Solid Gas = _________ Gas Solid = __________ Solid

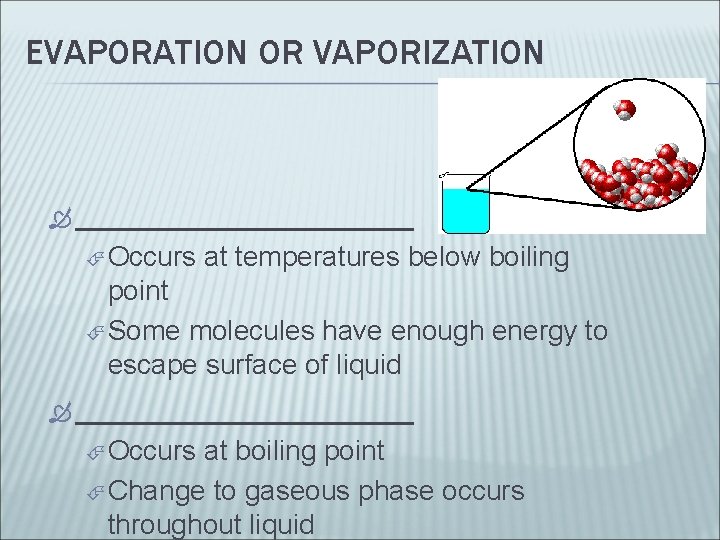
EVAPORATION OR VAPORIZATION __________ Occurs at temperatures below boiling point Some molecules have enough energy to escape surface of liquid __________ Occurs at boiling point Change to gaseous phase occurs throughout liquid

PHASE CHANGE (CONT) Freezing point and Melting point Same thing (occur at same temperature) Named depending on the direction compound is going Condensation point and Boiling point Same thing (occur at same temperature) Named depending on the direction compound is going Each substance has its own points and heats (fusion and vaporization)

INFLUENCING POINTS Same substance Pressure (mostly sways boiling point) ____ points are points at standard pressure (1 atm) Lower pressures allow particles to spread out more (IMF’s can be overcome at lower temps) Higher pressures compress molecules (Higher temp needed to overcome IMF’s) Between Strength different compounds of forces holding particles together Metallic Bonds Ionic Bonds Covalent Bonds IMF’s

BOILING POINT Vapor pressure- Partial pressure of gas particles of substance over the liquid of that substance Vapor pressure increases with temperature More Point particles have energy to escape surface at which vapor pressure of substance is equal to atmospheric pressure

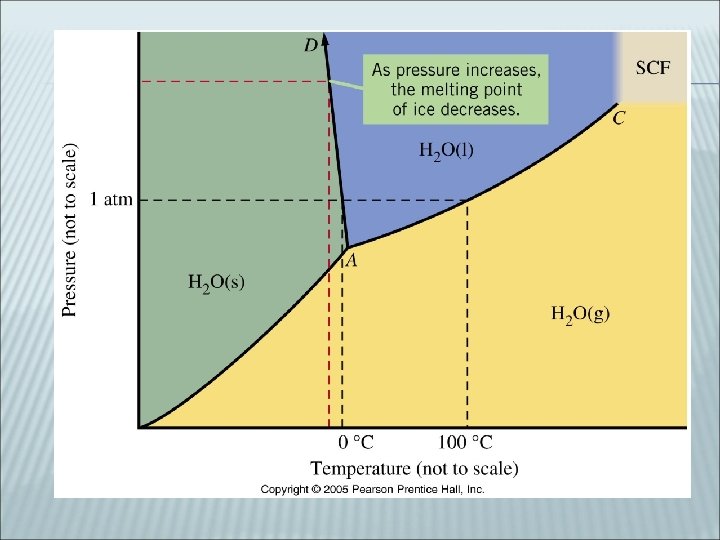
PHASE DIAGRAM Chart for each substance showing the temperature for phase changes according to pressure Crossing a line indicates a ________


PHASE DIAGRAMS (CONT) At any pressure, a horizontal line can be drawn. Temperatures of phase changes are found where lines are crossed The “normal” points are found by drawing a horizontal line at 1 atm of pressure


PHASE DIAGRAMS (CONT) _______ Pressure and Temperature where all three phases can be found ________ Critical Temperature- highest temperature that the liquid phase of a substance can be found Critical Pressure- pressure at critical point Beyond this point the liquid and gas phase in indistinguishable (super critical fluid)