Phases of Matter Phases of Matter can take





- Slides: 14

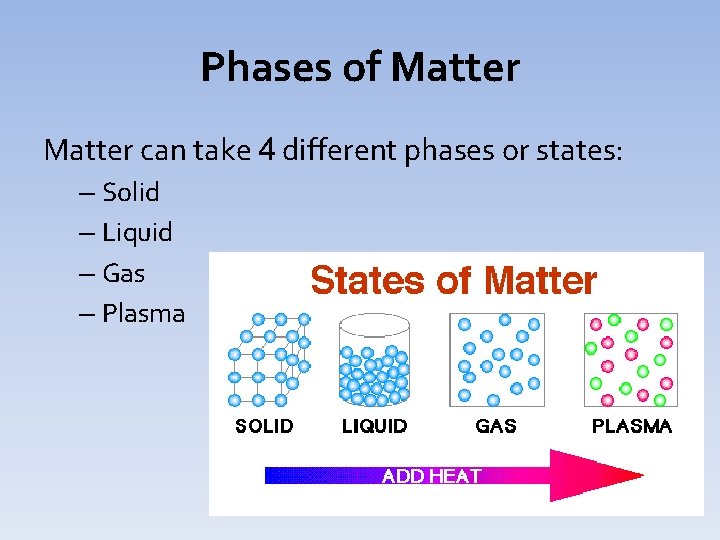
Phases of Matter

Phases of Matter can take 4 different phases or states: – Solid – Liquid – Gas – Plasma

Solids • Solids are substances that have a definite shape and a definite volume. • In other words, the shape of a solid never changes, nor does the space it takes up (volume. ) • The particles in a solid are packed tightly together so this is why its shape and volume don’t change.

2 Types of Solids 1. Crystalline Solids- particles are arranged in a repeating pattern. - These substances do NOT lose shape when heated. - Examples- sugar, sand, salt

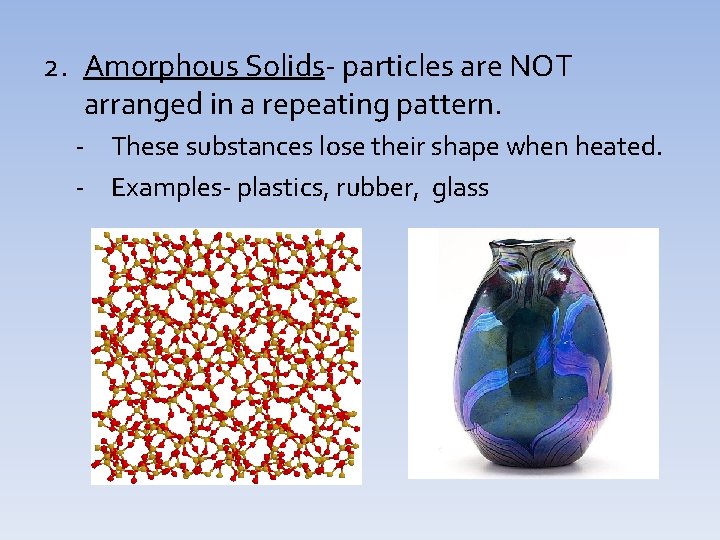
2. Amorphous Solids- particles are NOT arranged in a repeating pattern. - These substances lose their shape when heated. - Examples- plastics, rubber, glass

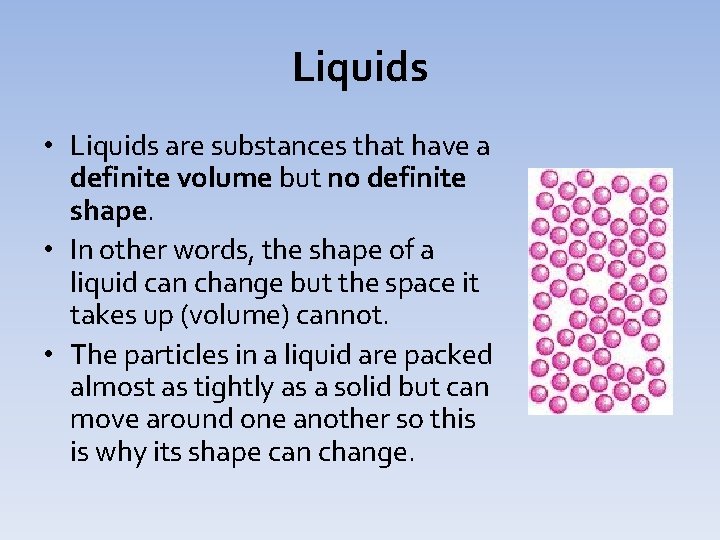
Liquids • Liquids are substances that have a definite volume but no definite shape. • In other words, the shape of a liquid can change but the space it takes up (volume) cannot. • The particles in a liquid are packed almost as tightly as a solid but can move around one another so this is why its shape can change.

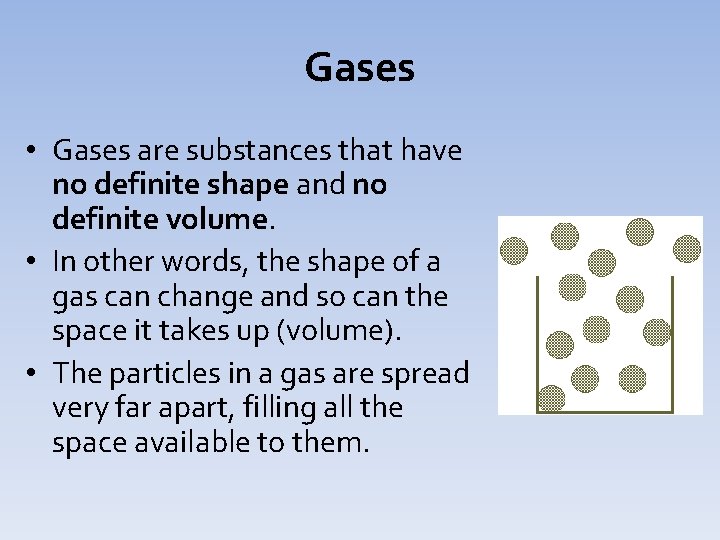
Gases • Gases are substances that have no definite shape and no definite volume. • In other words, the shape of a gas can change and so can the space it takes up (volume). • The particles in a gas are spread very far apart, filling all the space available to them.

Plasma • Plasma is a state of matter that occurs at very high temperatures. • It is too cold on Earth for most matter to reach the plasma state. • Our sun and small stars are made of plasma. • Lighting strikes are plasma and plasma glows when it conducts electricity in neon signs and fluorescent bulbs. • The hottest candle flame is plasma.

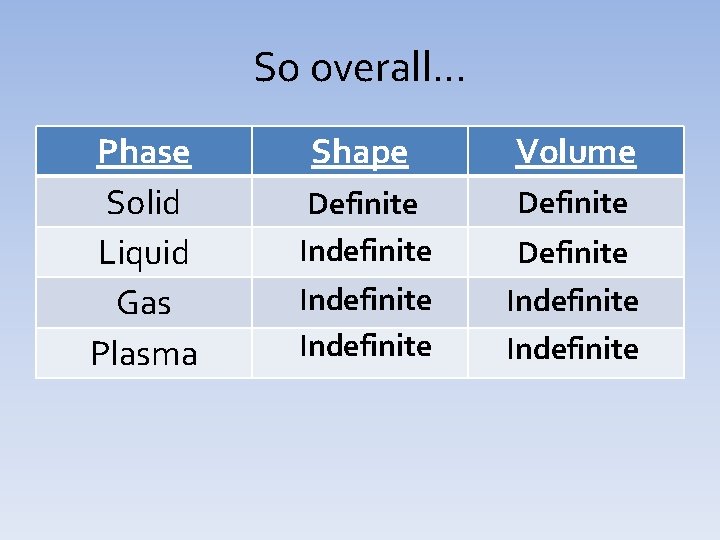
So overall… Phase Solid Liquid Gas Plasma Shape Volume Definite Indefinite Definite Indefinite

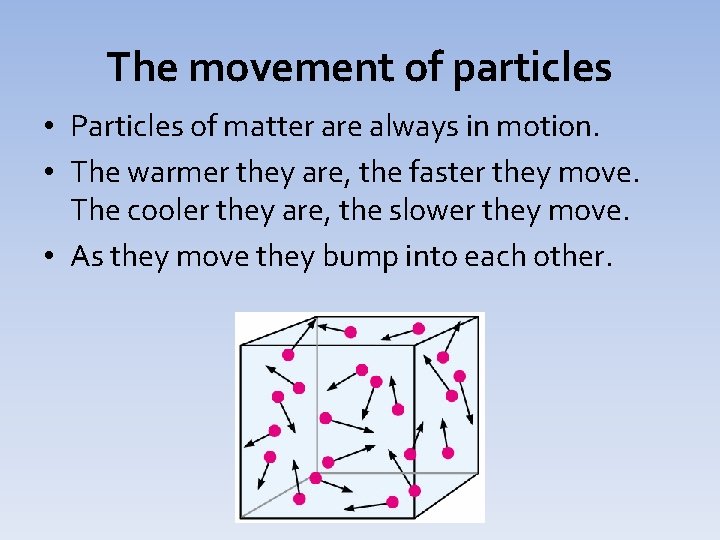
The movement of particles • Particles of matter are always in motion. • The warmer they are, the faster they move. The cooler they are, the slower they move. • As they move they bump into each other.

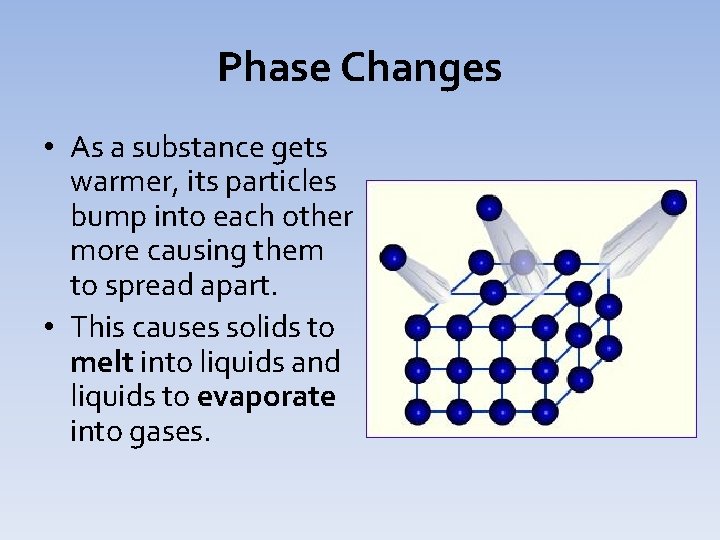
Phase Changes • As a substance gets warmer, its particles bump into each other more causing them to spread apart. • This causes solids to melt into liquids and liquids to evaporate into gases.

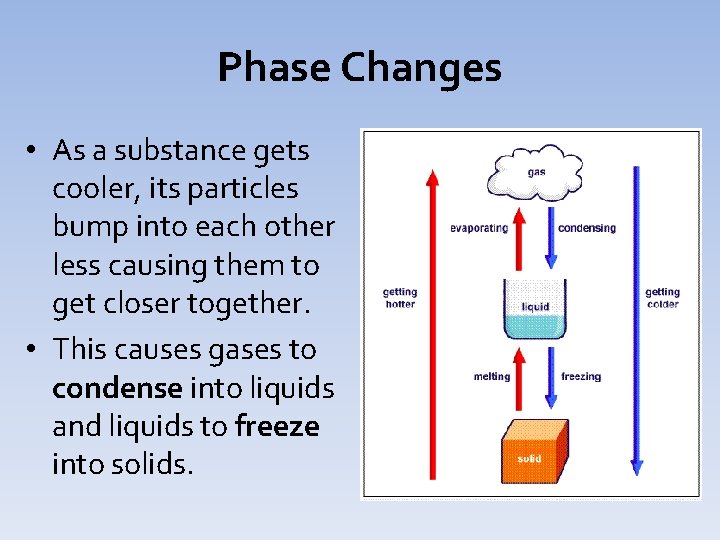
Phase Changes • As a substance gets cooler, its particles bump into each other less causing them to get closer together. • This causes gases to condense into liquids and liquids to freeze into solids.

S L Melting L S Freezing L G Evaporating G L Condensing

Sublimation • When a substance changes from a solid straight to a gas (skipping liquid), it sublimes. • S G • Only some substances can do this like dry ice and iodine.
 Take a bus or take a train
Take a bus or take a train Four states of matter
Four states of matter Four states of matter
Four states of matter 5 phases of matter
5 phases of matter 3 phases of matter
3 phases of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases States of matter foldable
States of matter foldable Phases of matter
Phases of matter Concept map of matter solid liquid and gas
Concept map of matter solid liquid and gas A typical programming tasks can be divided into two phases
A typical programming tasks can be divided into two phases What does ipde stand for?
What does ipde stand for? Where can i take forklift classes near me
Where can i take forklift classes near me Alive alexander pappas
Alive alexander pappas Can you take ventolin while pregnant
Can you take ventolin while pregnant Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter