Phases of Matter chapter 1314 1 Phases of



- Slides: 30

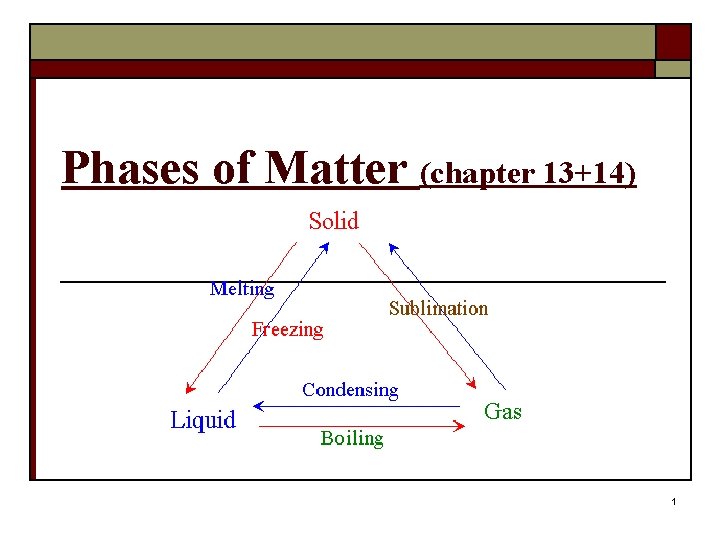
Phases of Matter (chapter 13+14) 1

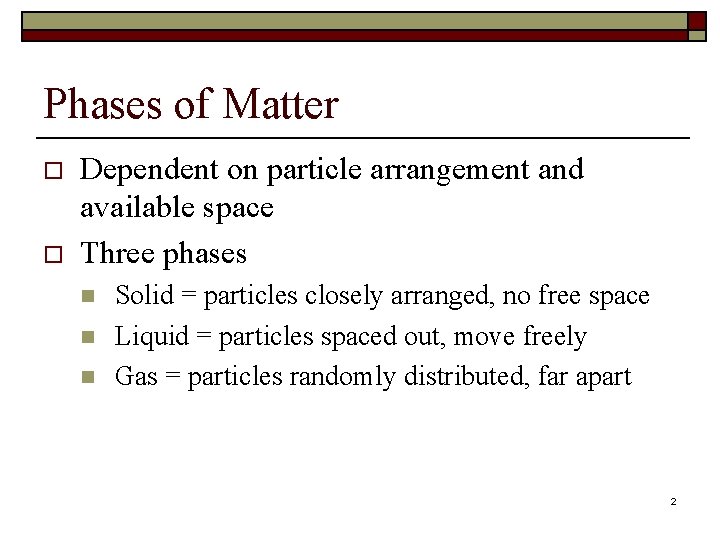
Phases of Matter o o Dependent on particle arrangement and available space Three phases n n n Solid = particles closely arranged, no free space Liquid = particles spaced out, move freely Gas = particles randomly distributed, far apart 2

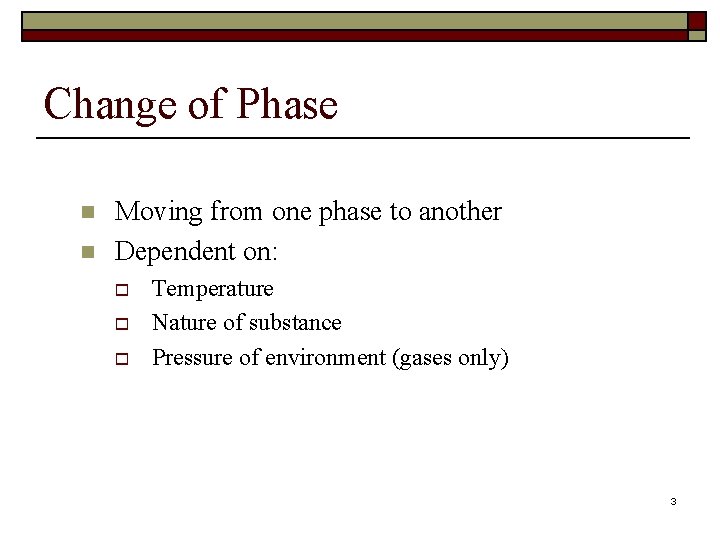
Change of Phase n n Moving from one phase to another Dependent on: o o o Temperature Nature of substance Pressure of environment (gases only) 3

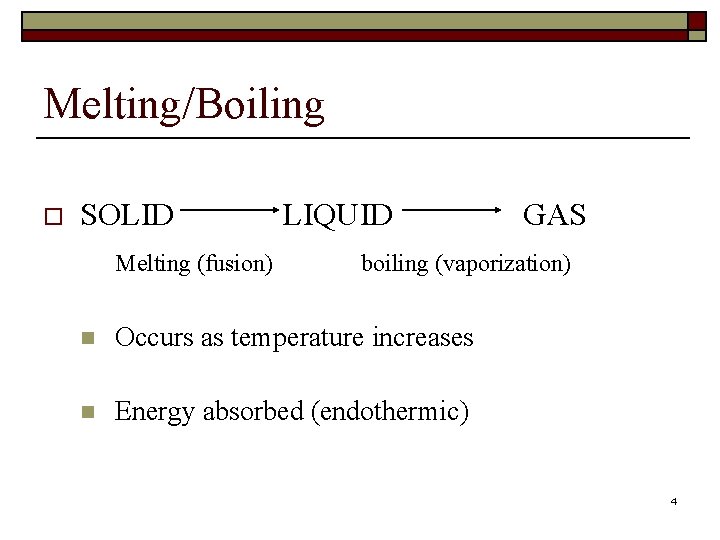
Melting/Boiling o SOLID Melting (fusion) LIQUID GAS boiling (vaporization) n Occurs as temperature increases n Energy absorbed (endothermic) 4

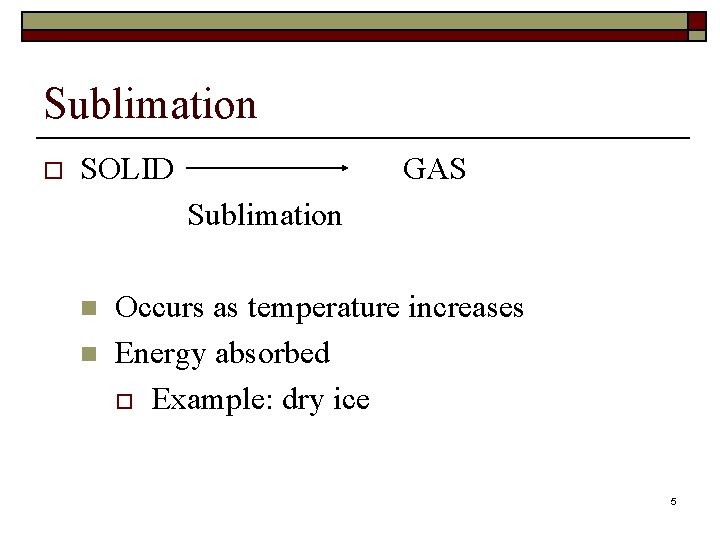
Sublimation o SOLID GAS Sublimation n n Occurs as temperature increases Energy absorbed o Example: dry ice 5

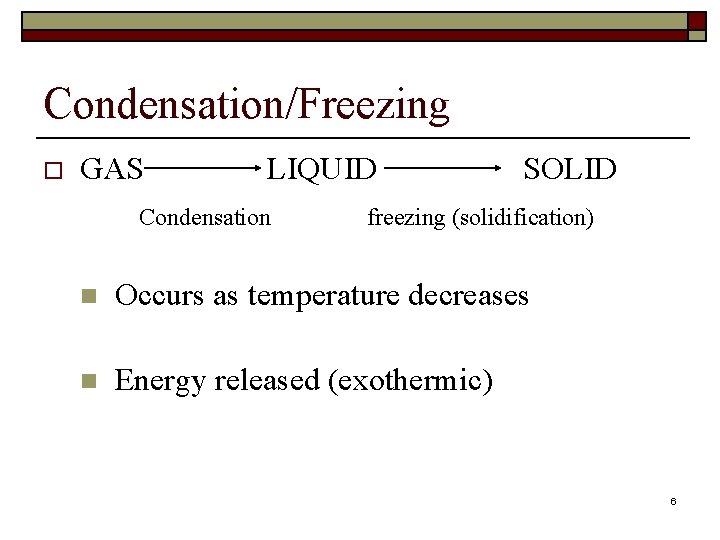
Condensation/Freezing o GAS LIQUID Condensation SOLID freezing (solidification) n Occurs as temperature decreases n Energy released (exothermic) 6

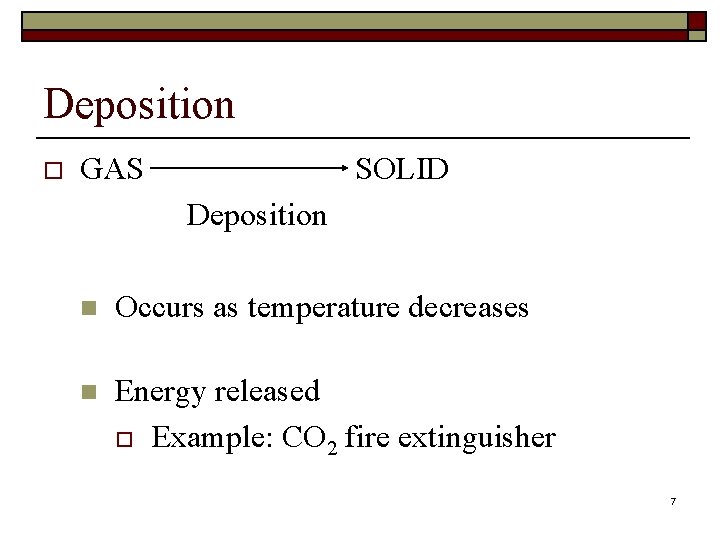
Deposition o GAS SOLID Deposition n Occurs as temperature decreases n Energy released o Example: CO 2 fire extinguisher 7

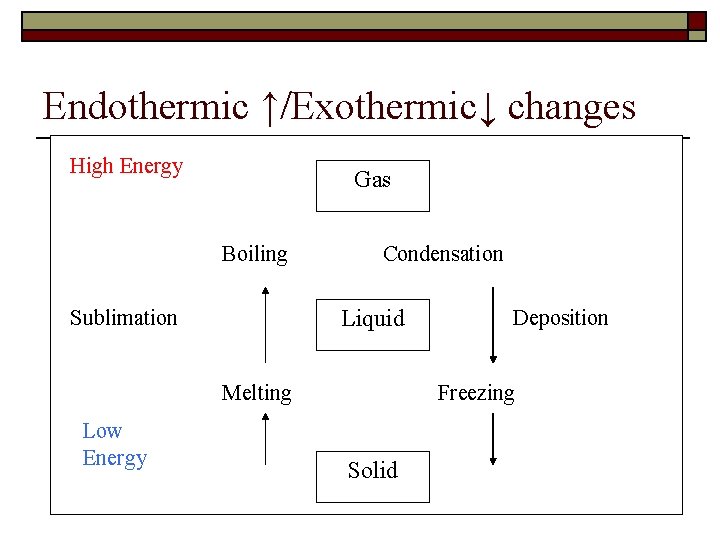
Endothermic ↑/Exothermic↓ changes High Energy Gas Boiling Sublimation Condensation Liquid Melting Low Energy Deposition Freezing Solid 8

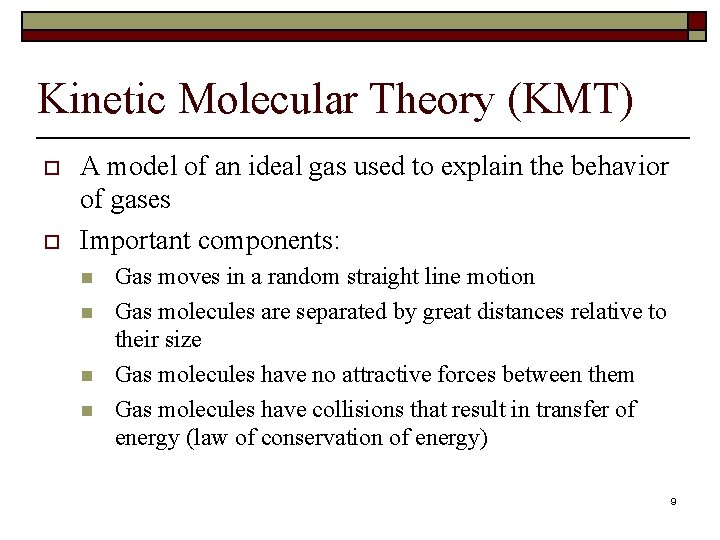
Kinetic Molecular Theory (KMT) o o A model of an ideal gas used to explain the behavior of gases Important components: n n Gas moves in a random straight line motion Gas molecules are separated by great distances relative to their size Gas molecules have no attractive forces between them Gas molecules have collisions that result in transfer of energy (law of conservation of energy) 9

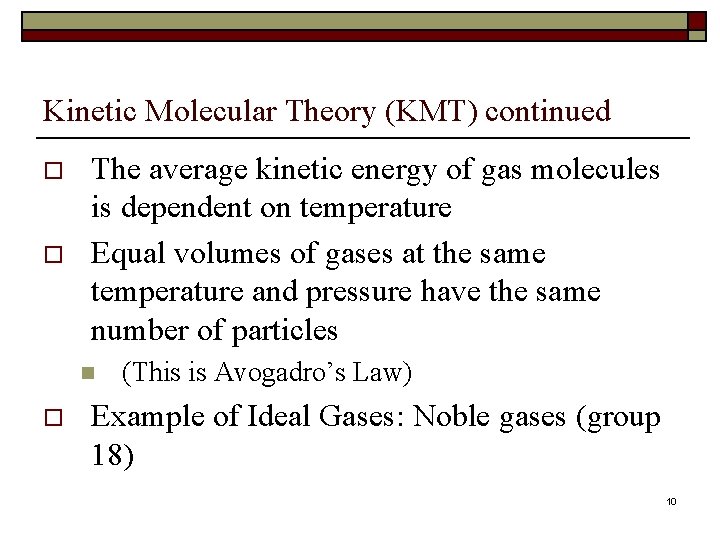
Kinetic Molecular Theory (KMT) continued o o The average kinetic energy of gas molecules is dependent on temperature Equal volumes of gases at the same temperature and pressure have the same number of particles n o (This is Avogadro’s Law) Example of Ideal Gases: Noble gases (group 18) 10

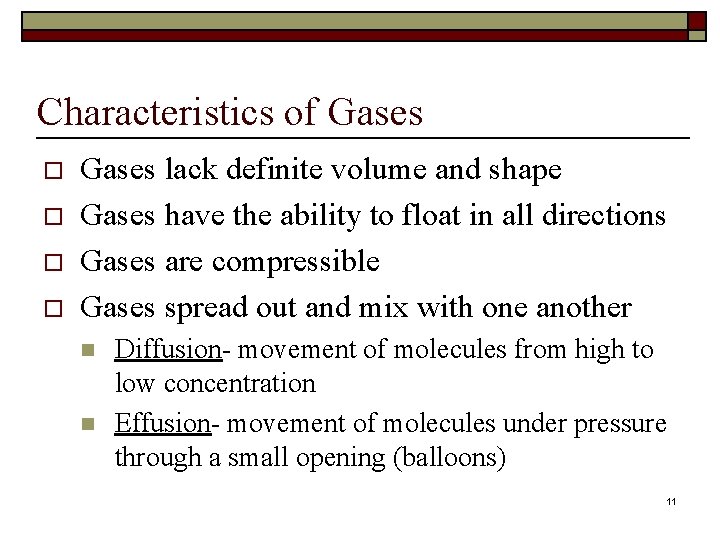
Characteristics of Gases o o Gases lack definite volume and shape Gases have the ability to float in all directions Gases are compressible Gases spread out and mix with one another n n Diffusion- movement of molecules from high to low concentration Effusion- movement of molecules under pressure through a small opening (balloons) 11

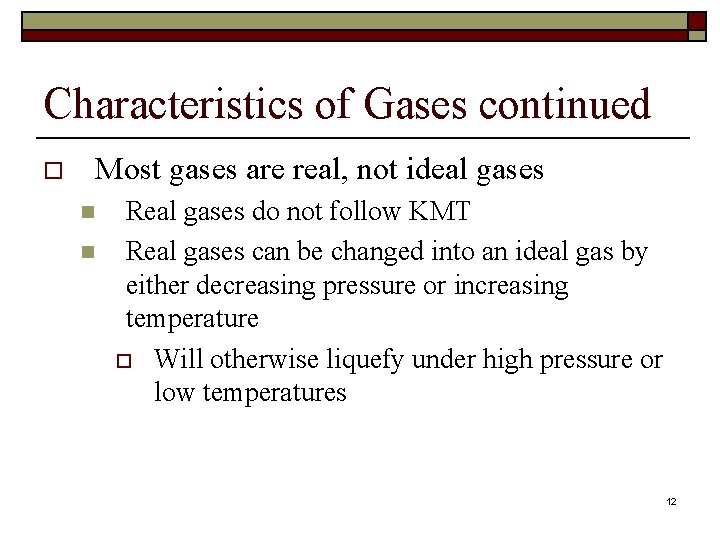
Characteristics of Gases continued o Most gases are real, not ideal gases n n Real gases do not follow KMT Real gases can be changed into an ideal gas by either decreasing pressure or increasing temperature o Will otherwise liquefy under high pressure or low temperatures 12

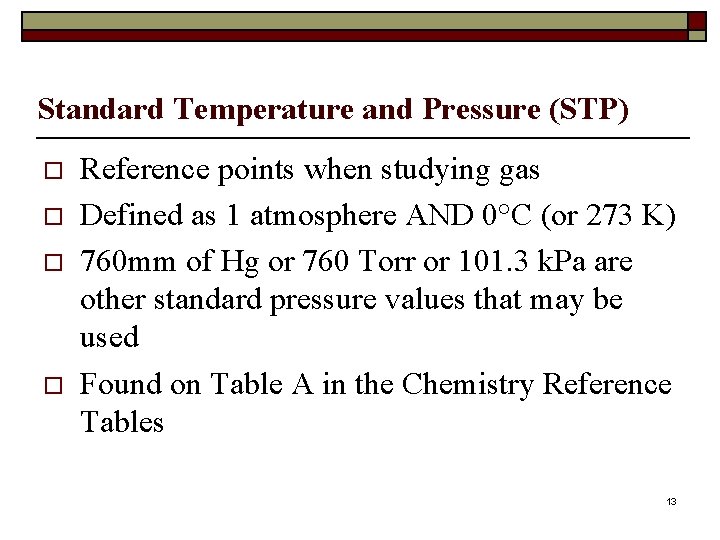
Standard Temperature and Pressure (STP) o o Reference points when studying gas Defined as 1 atmosphere AND 0°C (or 273 K) 760 mm of Hg or 760 Torr or 101. 3 k. Pa are other standard pressure values that may be used Found on Table A in the Chemistry Reference Tables 13

The Gas Laws MUST USE KELVIN TEMPS!!! o Simple mathematical relationships involving: n n o -volume -temperature -pressure -number of particles You will need to convert to K using the formula provided from Table T n K = °C + 273 14

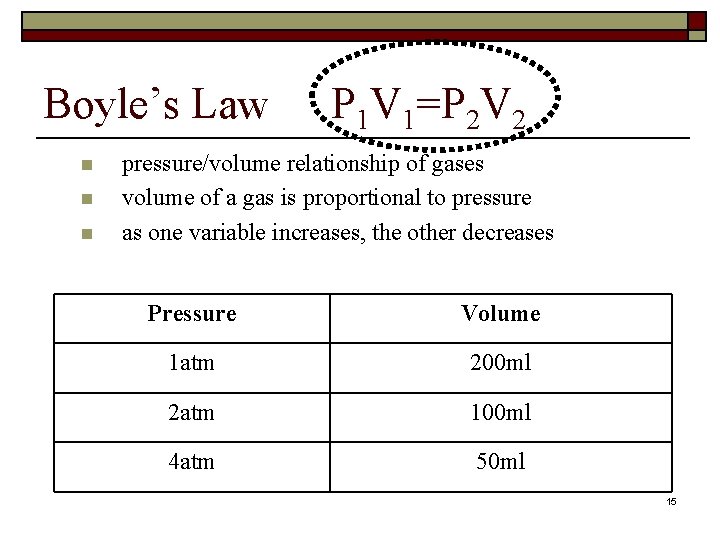
Boyle’s Law n n n P 1 V 1=P 2 V 2 pressure/volume relationship of gases volume of a gas is proportional to pressure as one variable increases, the other decreases Pressure Volume 1 atm 200 ml 2 atm 100 ml 4 atm 50 ml 15

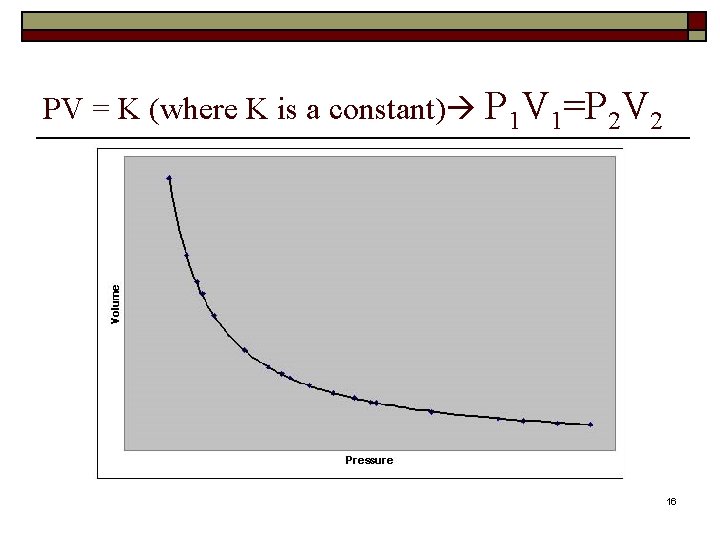
PV = K (where K is a constant) P 1 V 1=P 2 V 2 16

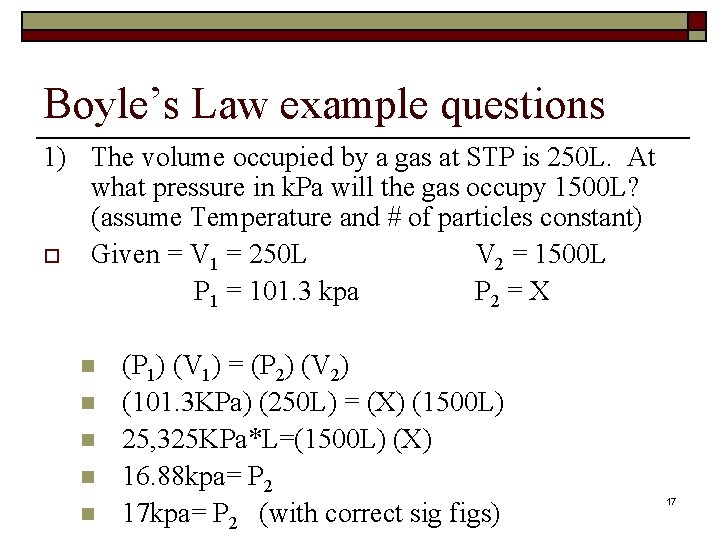
Boyle’s Law example questions 1) The volume occupied by a gas at STP is 250 L. At what pressure in k. Pa will the gas occupy 1500 L? (assume Temperature and # of particles constant) o Given = V 1 = 250 L V 2 = 1500 L P 1 = 101. 3 kpa P 2 = X n n n (P 1) (V 1) = (P 2) (V 2) (101. 3 KPa) (250 L) = (X) (1500 L) 25, 325 KPa*L=(1500 L) (X) 16. 88 kpa= P 2 17 kpa= P 2 (with correct sig figs) 17

Boyle’s Law example questions 2) A balloon with helium gas has a volume of 500 m. L at a pressure of 1 atm, The balloon reaches an altitude of 6. 5 km where the pressure is 0. 5 atm. Assuming the temperature hasn’t changed, what volume does the gas now occupy in the balloon? 18

Boyle’s Law example questions 3) A gas has a pressure of 1. 26 atm and occupies 7. 40 L. If the gas is compressed to 2. 93 L, what will its new pressure be, assuming constant temp? 19

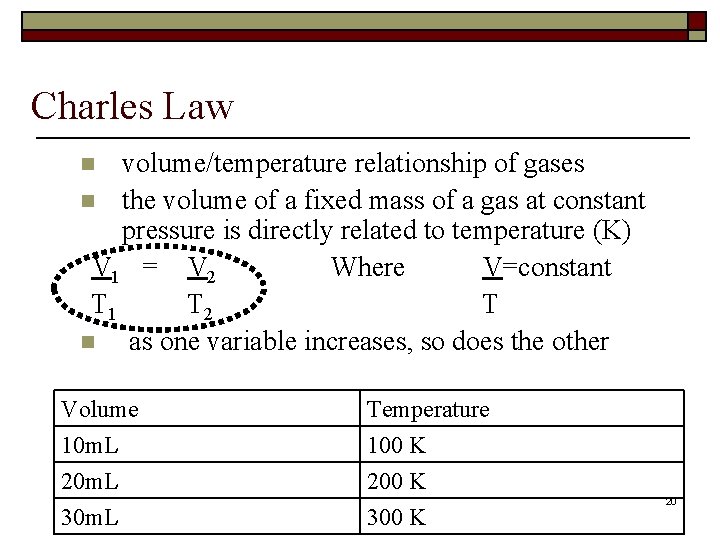
Charles Law volume/temperature relationship of gases n the volume of a fixed mass of a gas at constant pressure is directly related to temperature (K) V 1 = V 2 Where V=constant T 1 T 2 T n as one variable increases, so does the other n Volume 10 m. L 20 m. L 30 m. L Temperature 100 K 200 K 300 K 20

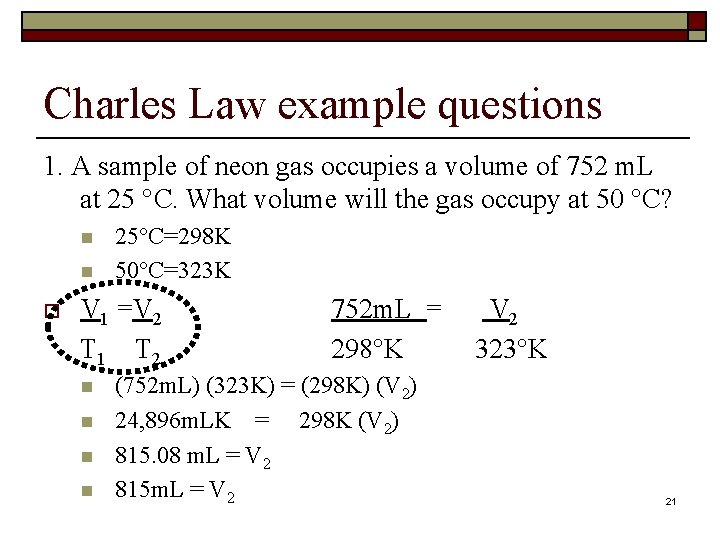
Charles Law example questions 1. A sample of neon gas occupies a volume of 752 m. L at 25 °C. What volume will the gas occupy at 50 °C? n n o 25°C=298 K 50°C=323 K V 1 =V 2 T 1 T 2 n n 752 m. L = 298°K (752 m. L) (323 K) = (298 K) (V 2) 24, 896 m. LK = 298 K (V 2) 815. 08 m. L = V 2 815 m. L = V 2 323°K 21

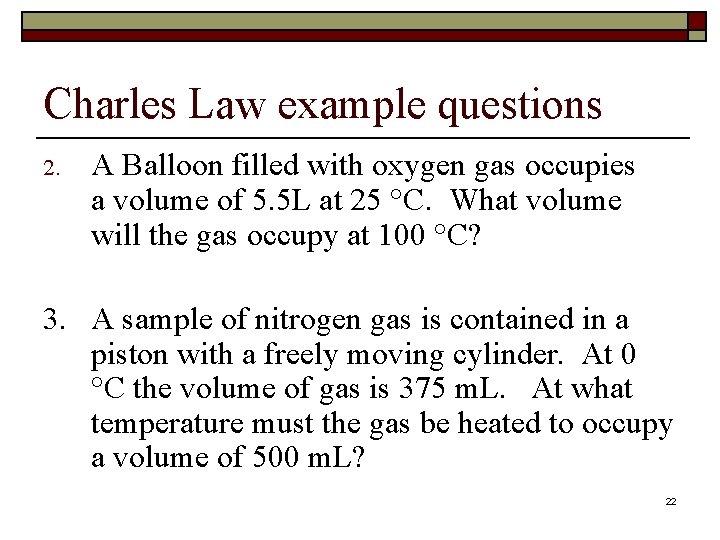
Charles Law example questions 2. A Balloon filled with oxygen gas occupies a volume of 5. 5 L at 25 °C. What volume will the gas occupy at 100 °C? 3. A sample of nitrogen gas is contained in a piston with a freely moving cylinder. At 0 °C the volume of gas is 375 m. L. At what temperature must the gas be heated to occupy a volume of 500 m. L? 22

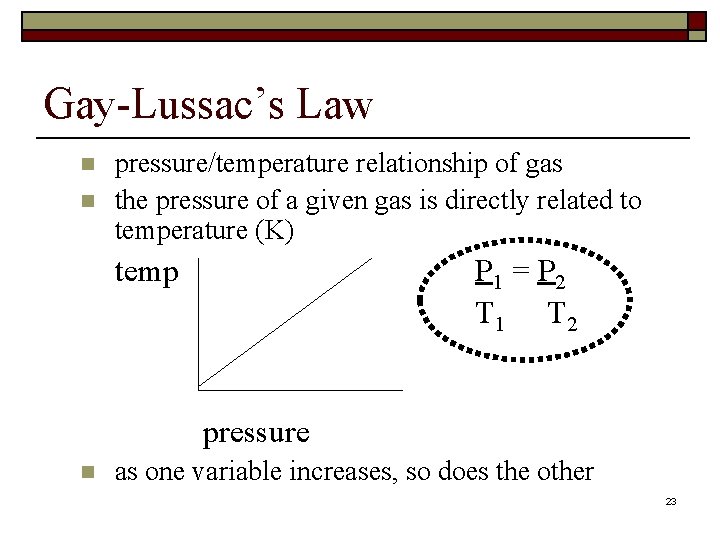
Gay-Lussac’s Law n n pressure/temperature relationship of gas the pressure of a given gas is directly related to temperature (K) temp P 1 = P 2 T 1 T 2 pressure n as one variable increases, so does the other 23

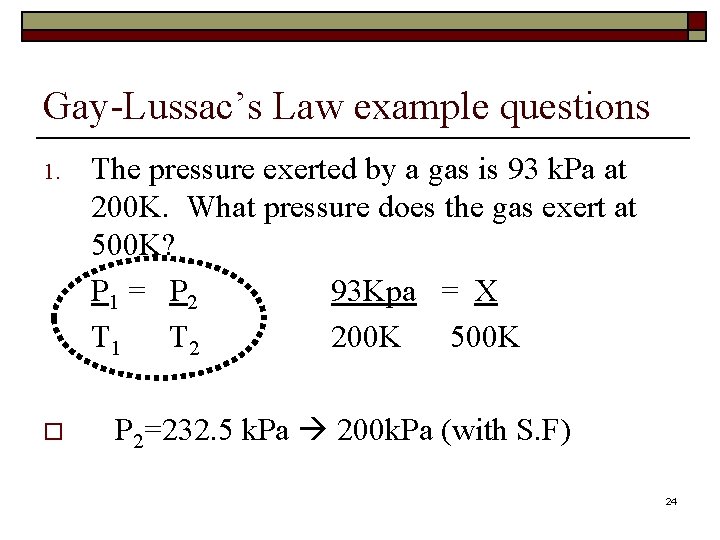
Gay-Lussac’s Law example questions 1. o The pressure exerted by a gas is 93 k. Pa at 200 K. What pressure does the gas exert at 500 K? P 1 = P 2 93 Kpa = X T 1 T 2 200 K 500 K P 2=232. 5 k. Pa 200 k. Pa (with S. F) 24

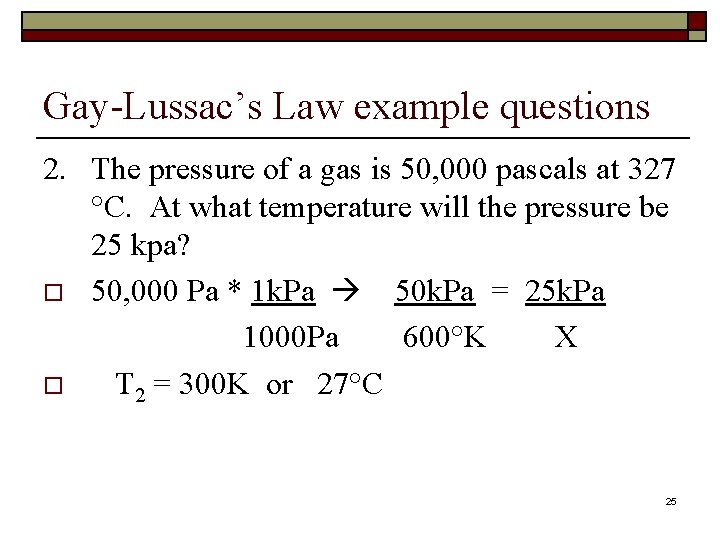
Gay-Lussac’s Law example questions 2. The pressure of a gas is 50, 000 pascals at 327 °C. At what temperature will the pressure be 25 kpa? o 50, 000 Pa * 1 k. Pa 50 k. Pa = 25 k. Pa 1000 Pa 600°K X o T 2 = 300 K or 27°C 25

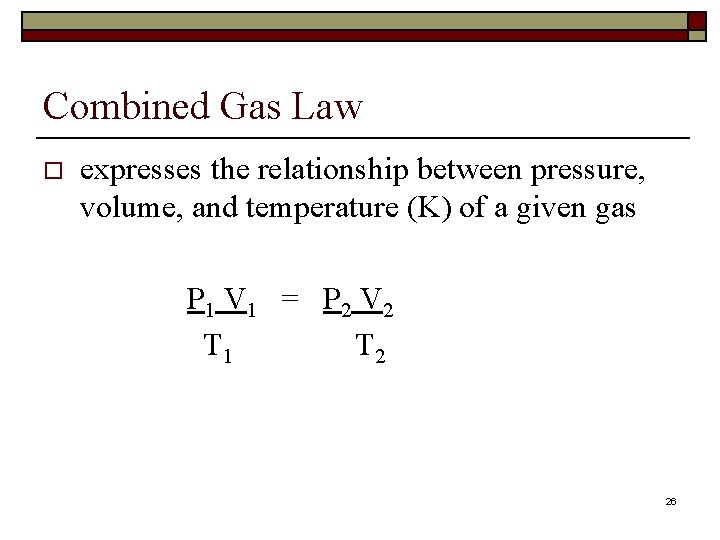
Combined Gas Law o expresses the relationship between pressure, volume, and temperature (K) of a given gas P 1 V 1 = P 2 V 2 T 1 T 2 26

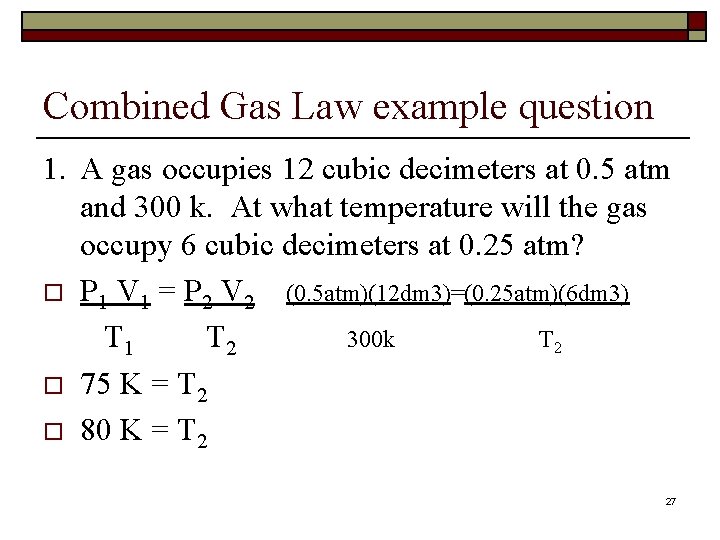
Combined Gas Law example question 1. A gas occupies 12 cubic decimeters at 0. 5 atm and 300 k. At what temperature will the gas occupy 6 cubic decimeters at 0. 25 atm? o P 1 V 1 = P 2 V 2 (0. 5 atm)(12 dm 3)=(0. 25 atm)(6 dm 3) T 1 T 2 300 k T 2 o 75 K = T 2 o 80 K = T 2 27

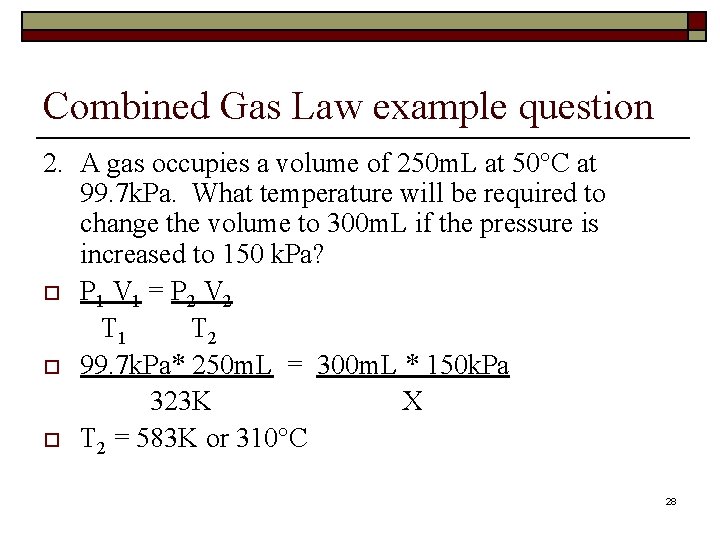
Combined Gas Law example question 2. A gas occupies a volume of 250 m. L at 50°C at 99. 7 k. Pa. What temperature will be required to change the volume to 300 m. L if the pressure is increased to 150 k. Pa? o P 1 V 1 = P 2 V 2 T 1 T 2 o 99. 7 k. Pa* 250 m. L = 300 m. L * 150 k. Pa 323 K X o T 2 = 583 K or 310°C 28

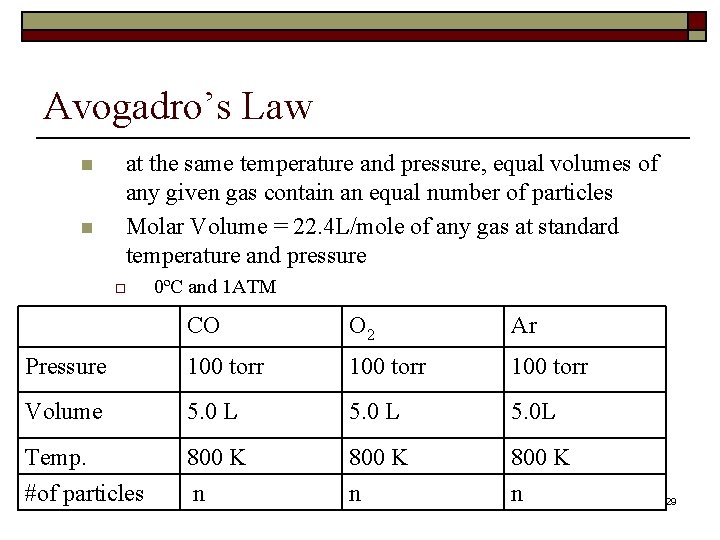
Avogadro’s Law n n at the same temperature and pressure, equal volumes of any given gas contain an equal number of particles Molar Volume = 22. 4 L/mole of any gas at standard temperature and pressure o 0ºC and 1 ATM CO O 2 Ar Pressure 100 torr Volume 5. 0 L 5. 0 L Temp. #of particles 800 K n 29

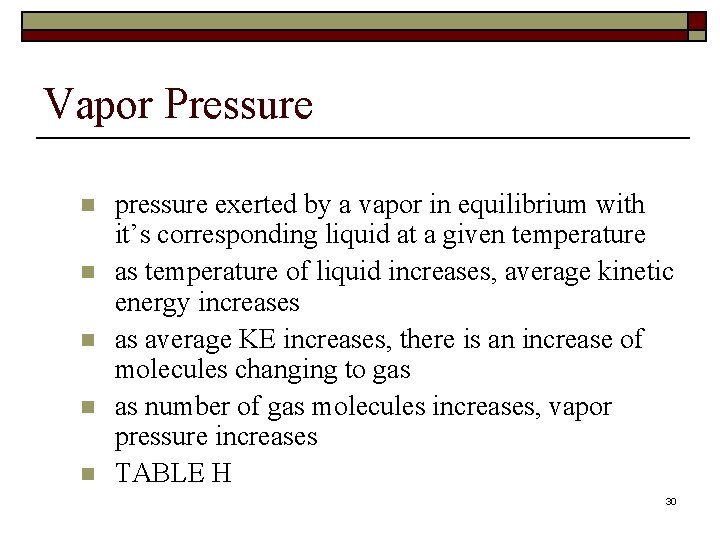
Vapor Pressure n n n pressure exerted by a vapor in equilibrium with it’s corresponding liquid at a given temperature as temperature of liquid increases, average kinetic energy increases as average KE increases, there is an increase of molecules changing to gas as number of gas molecules increases, vapor pressure increases TABLE H 30
 Math 1314
Math 1314 Solo hay dos caminos biblia
Solo hay dos caminos biblia 1314. matrix block sum
1314. matrix block sum Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Four states of matter
Four states of matter Four states of matter
Four states of matter 5 phases of matter
5 phases of matter 3 phases of matter
3 phases of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases States of matter foldable
States of matter foldable Phases of matter
Phases of matter Concept map matter
Concept map matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter White matter of brain
White matter of brain Section 1 composition of matter
Section 1 composition of matter Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter What is grey and white matter
What is grey and white matter Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chapter 1 review matter and change
Chapter 1 review matter and change Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 A matter of trust chapter questions and answers
A matter of trust chapter questions and answers 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key What is composition in matter
What is composition in matter Section 2 properties of matter
Section 2 properties of matter






















































