Phases of Matter Chapter 3 1 Matter and












- Slides: 25

Phases of Matter Chapter 3 1

Matter and Energy Section 1 2

Let’s Review! • Matter is… – Anything that has mass and takes up space – Can be a pure substance… • Element or compound – Or a mixture… • Homogeneous or heterogeneous – Made of… • Atoms and molecules 3

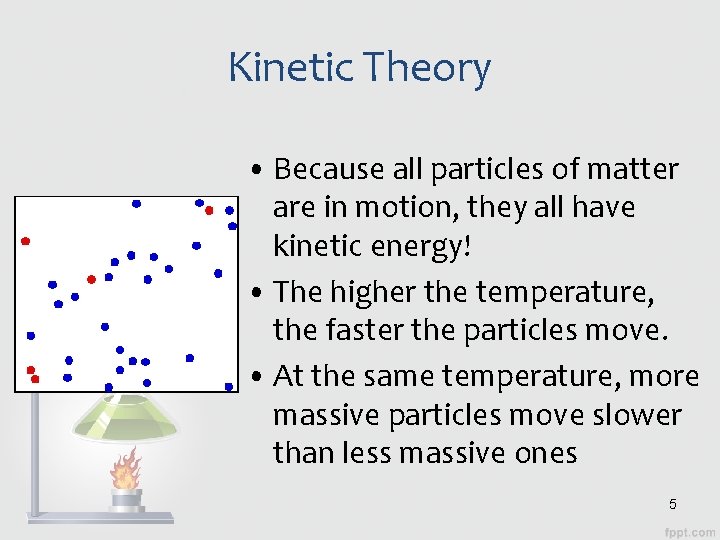
Kinetic Theory • Energy: the ability to change or move matter, or to do work. • Kinetic Energy: energy of motion • Kinetic Theory of Matter: – ALL matter is made of atoms and molecules – These atoms and molecules act like tiny particles that are always in motion 4

Kinetic Theory • Because all particles of matter are in motion, they all have kinetic energy! • The higher the temperature, the faster the particles move. • At the same temperature, more massive particles move slower than less massive ones 5

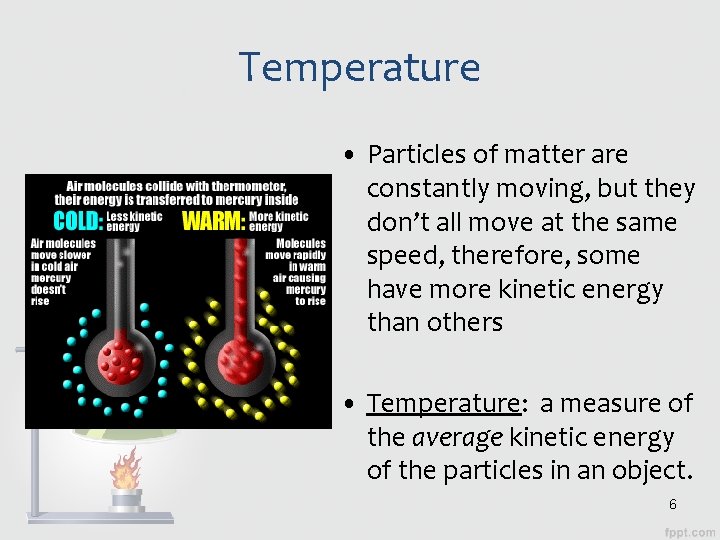
Temperature • Particles of matter are constantly moving, but they don’t all move at the same speed, therefore, some have more kinetic energy than others • Temperature: a measure of the average kinetic energy of the particles in an object. 6

Thermal Energy • Thermal Energy: total kinetic energy of the particles that make up a substance • Temperature versus thermal energy – Temperature is average kinetic energy – Thermal energy is determined by the amount of a substance 7

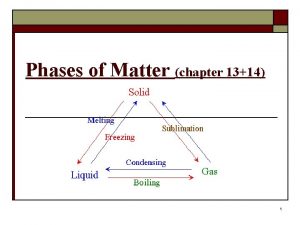
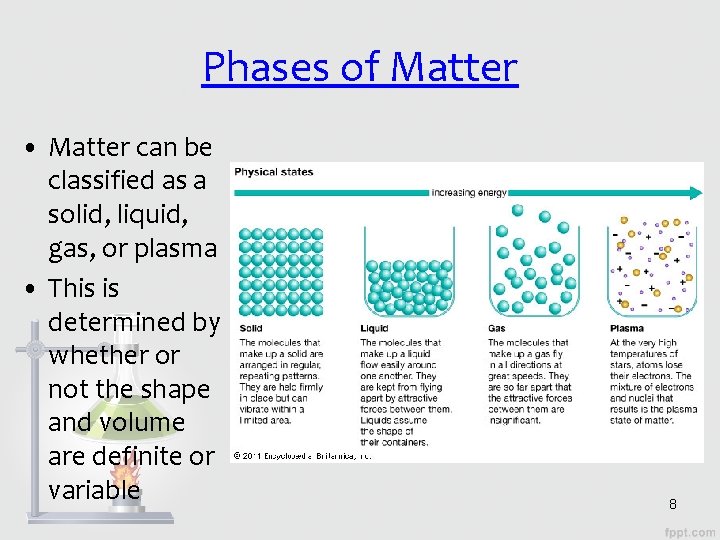
Phases of Matter • Matter can be classified as a solid, liquid, gas, or plasma • This is determined by whether or not the shape and volume are definite or variable 8

Solid • Solid: atoms/molecules can only vibrate in one place, occasionally slide by one another. – students in a detention fidgeting in their seats • Shape: definite • Volume: definite • Energy: low • Crystalline Solid: atoms arranged in a regular repeating pattern – Salt, diamonds • Amorphous Solid: No repeating pattern – Wax, glass 9

Liquid • Liquid: atoms/molecules can move about freely – students in the cafeteria • Shape: variable • Volume: definite • Energy: mid-level • Surface Tension: Attraction of molecules at the surface of a liquid – this causes liquids to form spherical drops • Viscosity: resistance to the flow of a liquid – low = runny – high = thicker, like honey 10

Gas • Gas: atoms/molecules move around wildly constantly running into each other. – football practice • Shape: variable • Volume: variable • Energy: High • Because the particles in liquids and gases can move past each other they are considered “fluids” 11

Plasma • 4 th and most common phase of matter in the universe • Shape: variable • Volume: variable • Energy: highest (the particles are electrically charged) • Affected by magnetic fields 12

Plasma • They act like gases but have some differences – plasma can conduct electric current while gases do not • Commonly found in stars, can be created naturally by lightning on Earth and in the hottest part of a very hot flame. 13

Changes of State Section 2 14

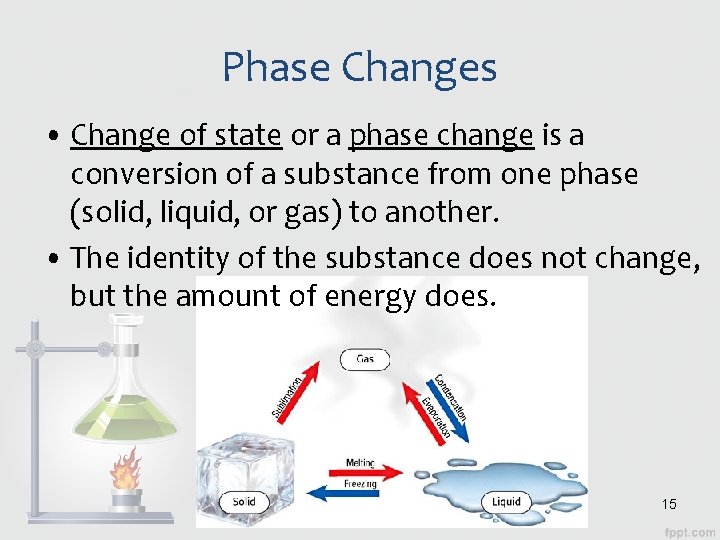
Phase Changes • Change of state or a phase change is a conversion of a substance from one phase (solid, liquid, or gas) to another. • The identity of the substance does not change, but the amount of energy does. 15

Phase Changes • Law of Conservation of Mass: Mass cannot be created or destroyed, it is conserved for all physical and chemical changes • Law of Conservation of Energy: Energy cannot be created or destroyed • Energy can change forms during physical or chemical changes, but the total amount present before and after is the same – IF energy of a substance changes it is because it came from another source! 16

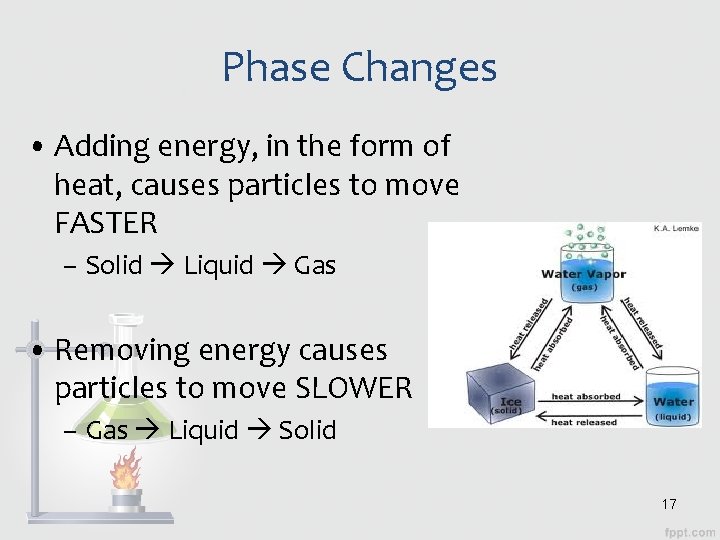
Phase Changes • Adding energy, in the form of heat, causes particles to move FASTER – Solid Liquid Gas • Removing energy causes particles to move SLOWER – Gas Liquid Solid 17

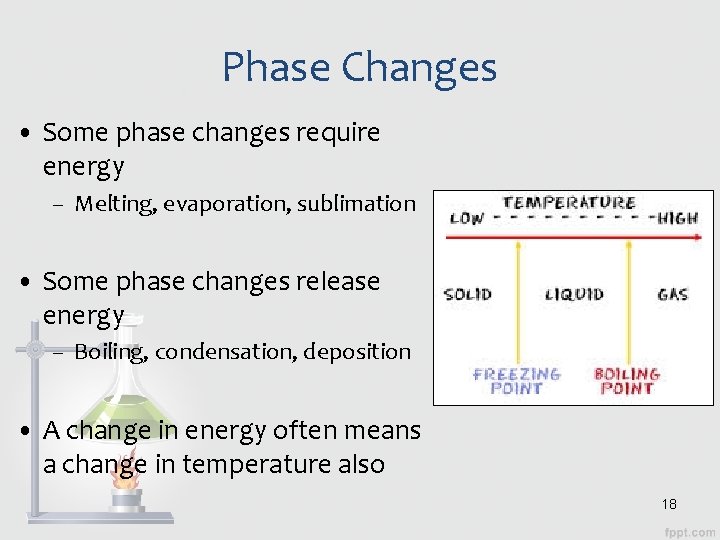
Phase Changes • Some phase changes require energy – Melting, evaporation, sublimation • Some phase changes release energy – Boiling, condensation, deposition • A change in energy often means a change in temperature also 18

Melting • Solid Liquid • Melting Point: the temperature at which a substance changes from solid to liquid. • Melting point depends on the pressure. 19

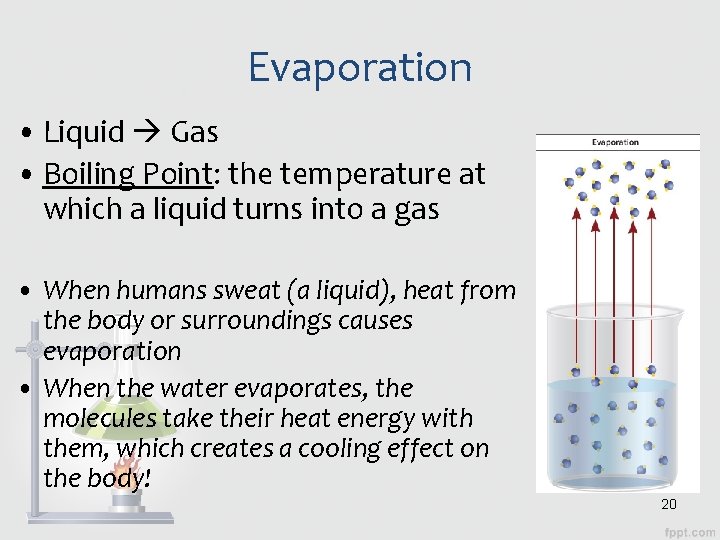
Evaporation • Liquid Gas • Boiling Point: the temperature at which a liquid turns into a gas • When humans sweat (a liquid), heat from the body or surroundings causes evaporation • When the water evaporates, the molecules take their heat energy with them, which creates a cooling effect on the body! 20

Sublimation • Solid Gas – Solid carbon dioxide (dry ice) goes from solid to gas – Ice cubes can go from ice to gas while still in the freezer 21

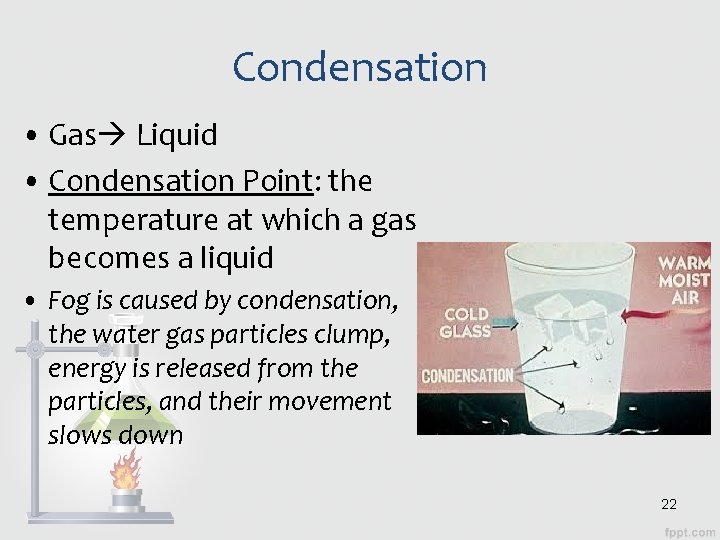
Condensation • Gas Liquid • Condensation Point: the temperature at which a gas becomes a liquid • Fog is caused by condensation, the water gas particles clump, energy is released from the particles, and their movement slows down 22

Freezing • Liquid Solid • Freezing Point: temperature at which a liquid becomes a solid 23

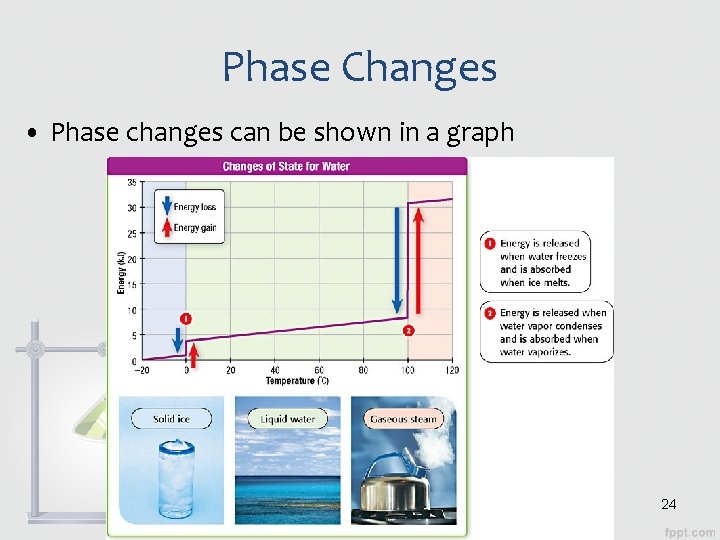
Phase Changes • Phase changes can be shown in a graph 24

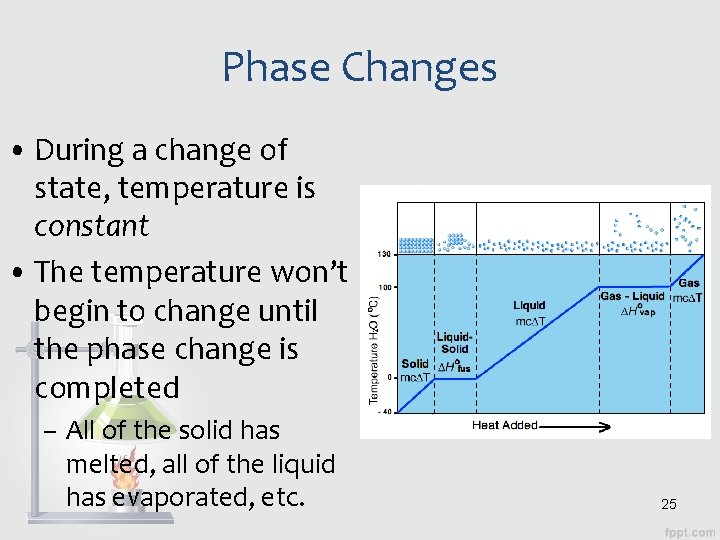
Phase Changes • During a change of state, temperature is constant • The temperature won’t begin to change until the phase change is completed – All of the solid has melted, all of the liquid has evaporated, etc. 25
 Four states of matter
Four states of matter 4 phases of matter
4 phases of matter 5 phases of matter
5 phases of matter 3 phases of matter
3 phases of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases States of matter foldable
States of matter foldable Phases of matter
Phases of matter Matter concept map
Matter concept map Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Grey matter of nervous system
Grey matter of nervous system Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Gray matter and white matter
Gray matter and white matter Ncl. caudatus
Ncl. caudatus Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Flow energy review
Flow energy review Lesson 2 moon phases and eclipses answer key
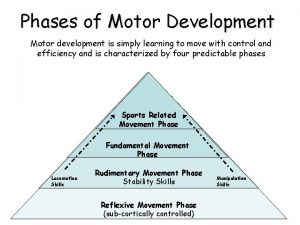
Lesson 2 moon phases and eclipses answer key Phases and stages of motor development
Phases and stages of motor development Anatomy of throwing a dart
Anatomy of throwing a dart Stress curve and phases
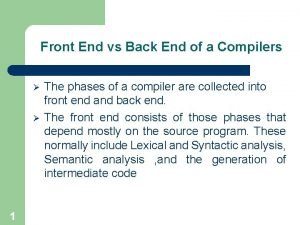
Stress curve and phases Front and back end of compiler
Front and back end of compiler Pulmonary ventilation consists of two cyclic phases,
Pulmonary ventilation consists of two cyclic phases, New moon facts
New moon facts When to use sdlc
When to use sdlc Moon phases and eclipses
Moon phases and eclipses