Properties of Matter Chemistry Mr Danahy Nanuet Senior














- Slides: 30

Properties of Matter Chemistry Mr. Danahy Nanuet Senior High School

What is Matter? Matter – anything that has mass and volume. Mass - a measurement of the amount of matter in an object. - Mass is not affected by location or gravity. - Mass of object remains the same even in different locations. Mass = Weight - Weight is an amount of force due to gravity and mass (F = mass x gravity), and varies depending on location (i. e. , gravity).

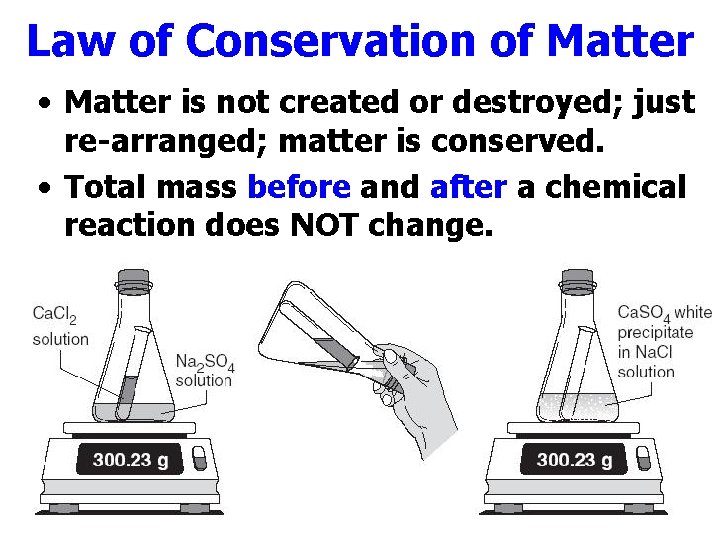
Law of Conservation of Matter • Matter is not created or destroyed; just re-arranged; matter is conserved. • Total mass before and after a chemical reaction does NOT change.

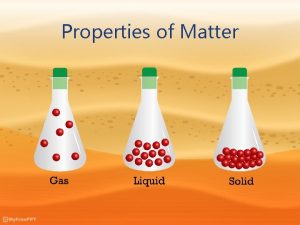
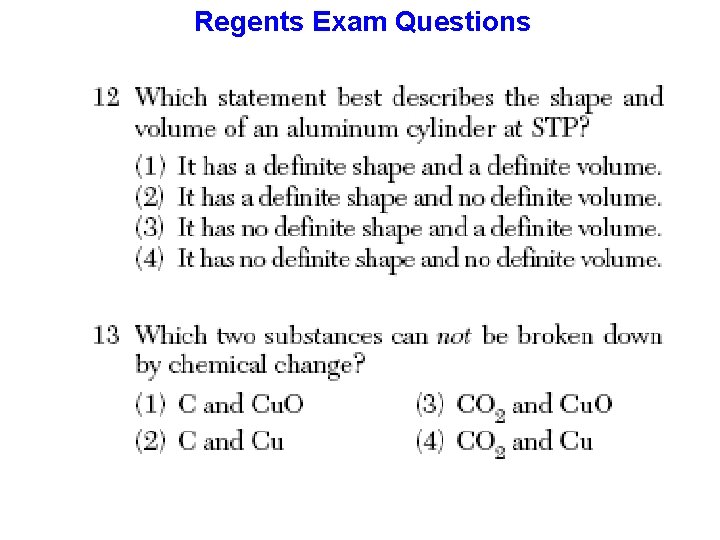
Four (4) States of Matter SOLID - BOTH A DEFINITE VOLUME and a DEFINITE SHAPE. A rock is a solid, solid matter can be picked up and carried around without having to place it in a special container. LIQUID - DEFINITE VOLUME but NO DEFINITE SHAPE. A key property of a liquid is that they FLOW and can be POURED.

Four (4) States of Matter GAS - NO DEFINITE VOLUME and NO DEFINITE SHAPE. A gas ALWAYS TAKES BOTH THE VOLUME AND THE SHAPE OF ANY CONTAINER INTO WHICH IT IS PLACED. If a gas is NOT in a container, it will spread out as far as it can. PLASMA - NO DEFINITE VOLUME OR SHAPE AND IS COMPOSED OF ELECTRICALLY CHARGED PARTICLES.

= Phases of Matter Properties of Matter Chemistry Mr. Danahy Expands to fill. Nanuet Senior High School No definite shape. Definite shape. No definite volume. Definite volume.

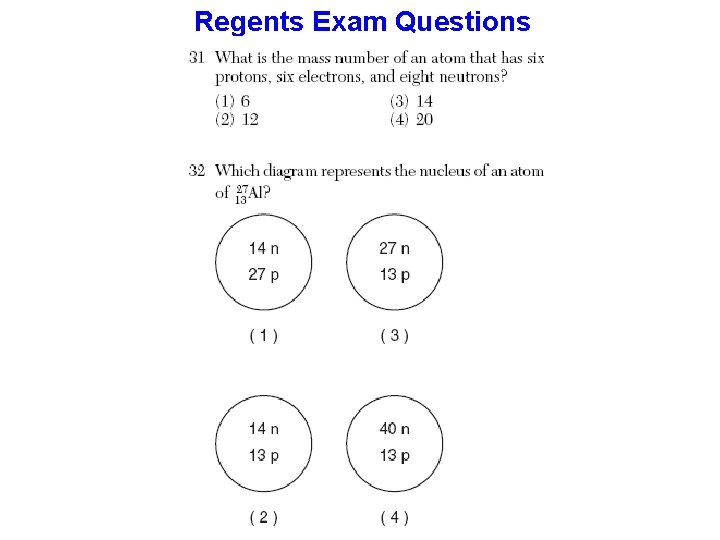
Properties of Matter TEMPERATURE – a measurement of the Average Kinetic Energy (KE) Temp. ~ Ave. KE - Temp. is NOT a form of energy - If Temp. increases, Ave. KE increases - Absolute Zero = - 273 C = 0 Kelvin = no motion KINETIC ENERGY – energy of motion of particles PARTICLES = atoms for elements Or = molecules for compounds

Pressure Measurement PRESSURE – a measurement of FORCE PER UNIT AREA. Air pressure is measured with a barometer. Examples of units: - Pounds per square inch (PSI) - 1 atmosphere (atm) = pressure at sea level - 1 atm = 760 mm Hg = 760 torr - 1 atm = 101. 3 kilopascals (k. Pa) See Ref. Table A

Kinetic Theory of Gases 1) Gas particles are small, hard spheres, with insignificant volume 2) Gas particles motion = in straight line paths, rapid, random directions 3) Gas particle collisions are perfectly elastic; no attraction between particles; no loss in energy; temp. (~ave. KE) remains constant

Properties of Matter Animations Why does Salt melt Ice? http: //antoine. frostburg. edu/chem/senese/101/solutions/faq/whysalt-melts-ice. shtml Phases of Matter visualization Gas Particle Motion visualization http: //intro. chem. okstate. edu/1314 F 00/Laboratory/GLP. htm

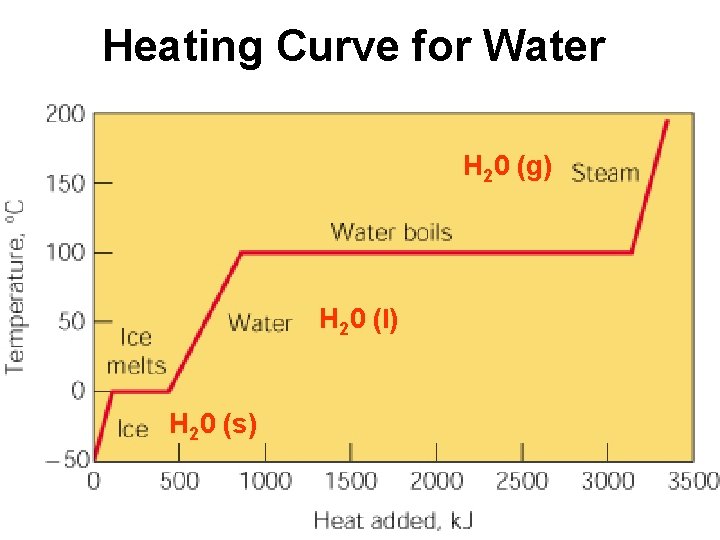
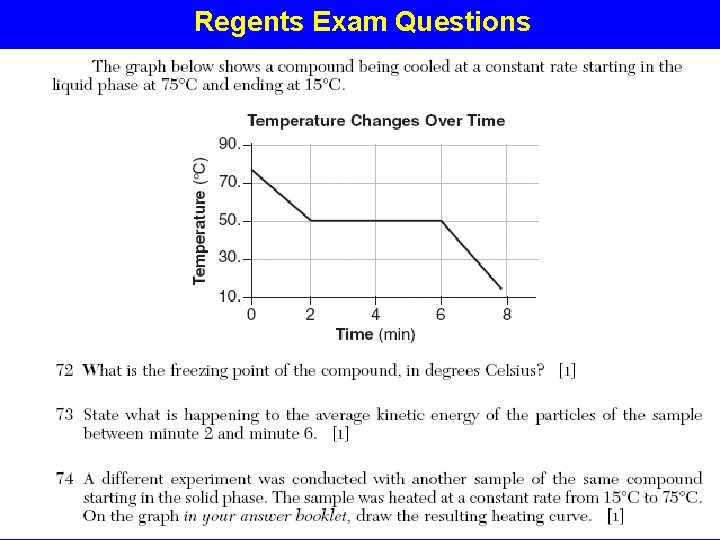
Physical Properties of Matter BOILING POINT - temp. a substance changes from liquid to gas. CONDENSATION POINT – temp. a substance changes from gas to liquid; same temperature as boiling point. DENSITY - The mass of a specific volume of substance. FREEZING POINT – temp. a substance changes from liquid to solid; same temperature as Melting Point. SOLUBILITY - The degree to which a substance will dissolve in a given amount of another substance, such as water.

Heating Curve for Water H 20 (g) H 20 (l) H 20 (s)


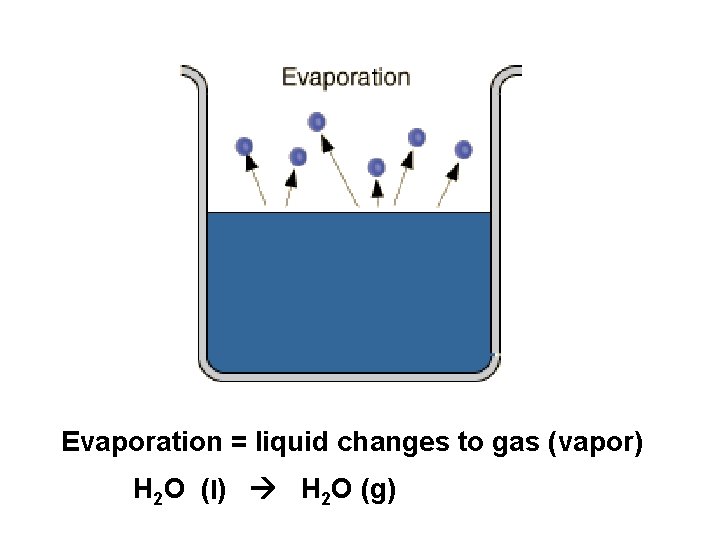
Evaporation = liquid changes to gas (vapor) H 2 O (l) H 2 O (g)

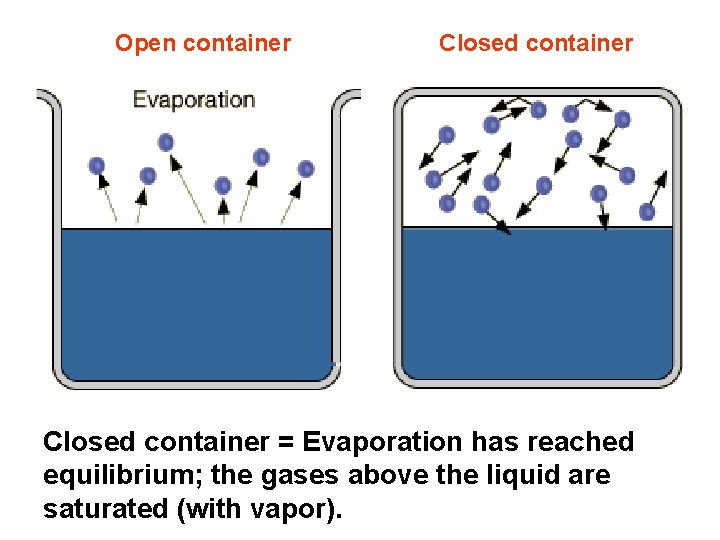
Open container Closed container = Evaporation has reached equilibrium; the gases above the liquid are saturated (with vapor).

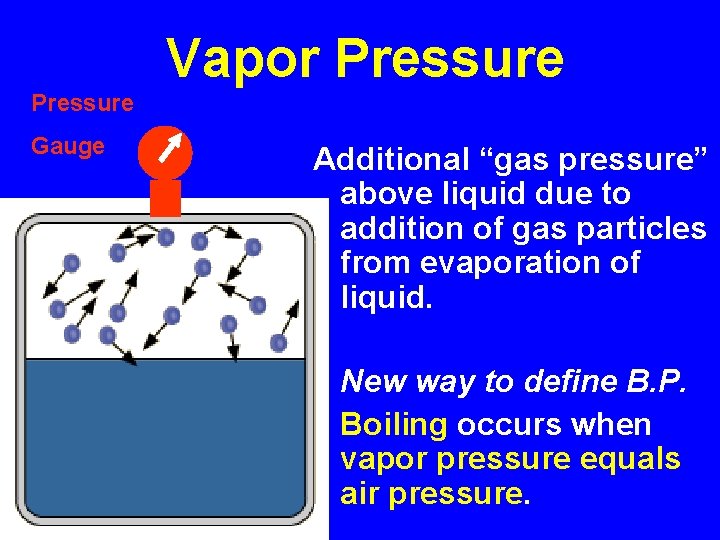
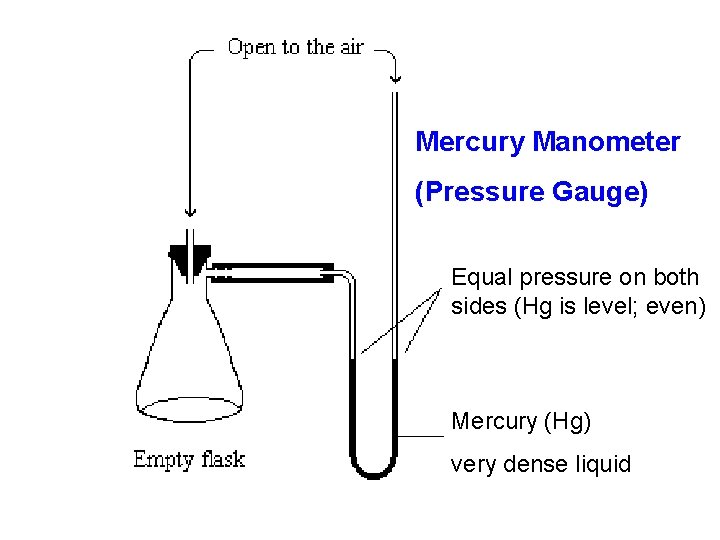
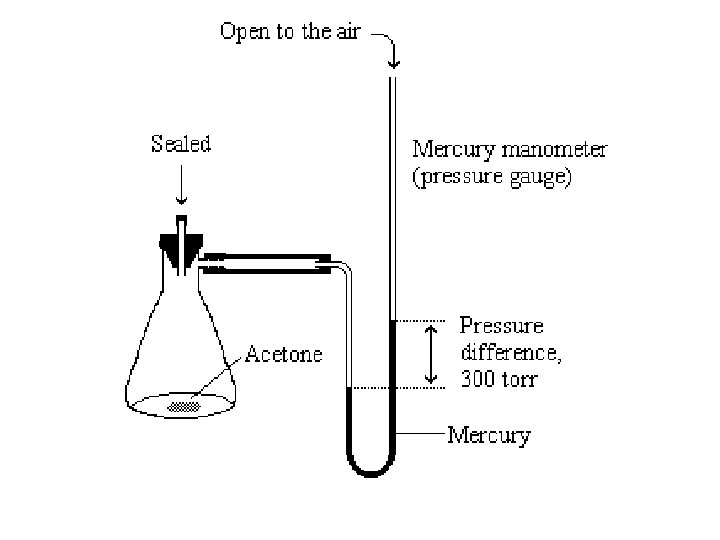
Vapor Pressure Gauge Additional “gas pressure” above liquid due to addition of gas particles from evaporation of liquid. New way to define B. P. Boiling occurs when vapor pressure equals air pressure.

Mercury Manometer (Pressure Gauge) Equal pressure on both sides (Hg is level; even) Mercury (Hg) very dense liquid



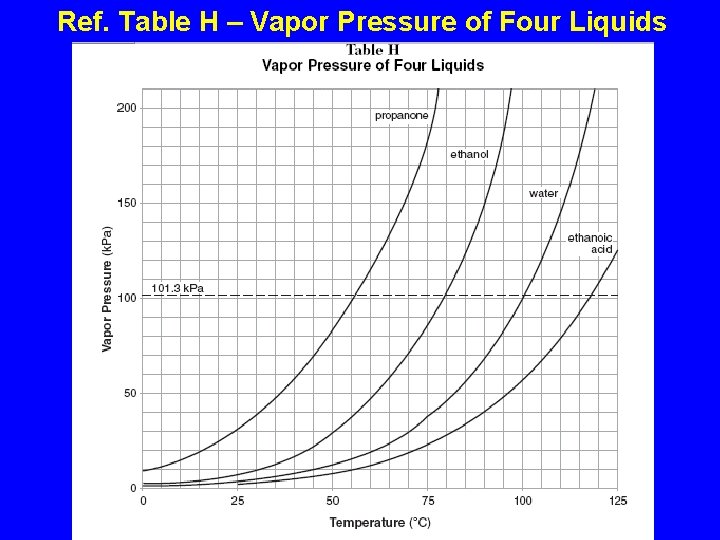
Ref. Table H – Vapor Pressure of Four Liquids

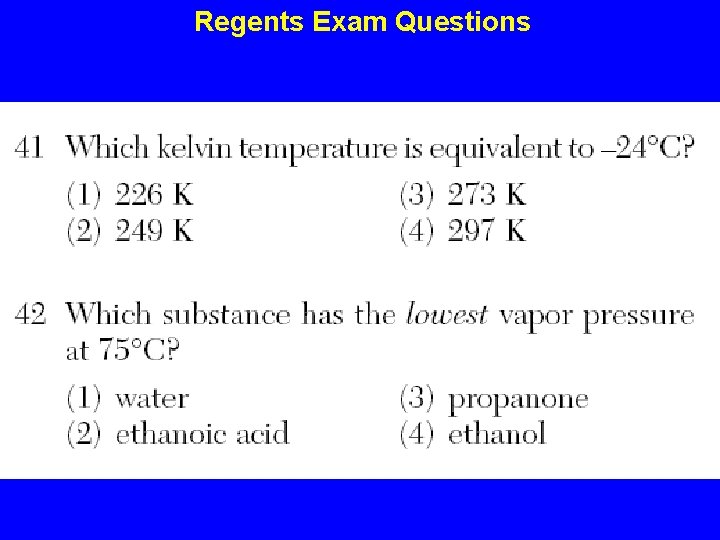
Regents Exam Questions

Regents Exam Questions

Regents Exam Questions

Regents Exam Questions

Regents Exam Questions

Regents Exam Questions

Regents Exam Questions

Regents Exam Questions


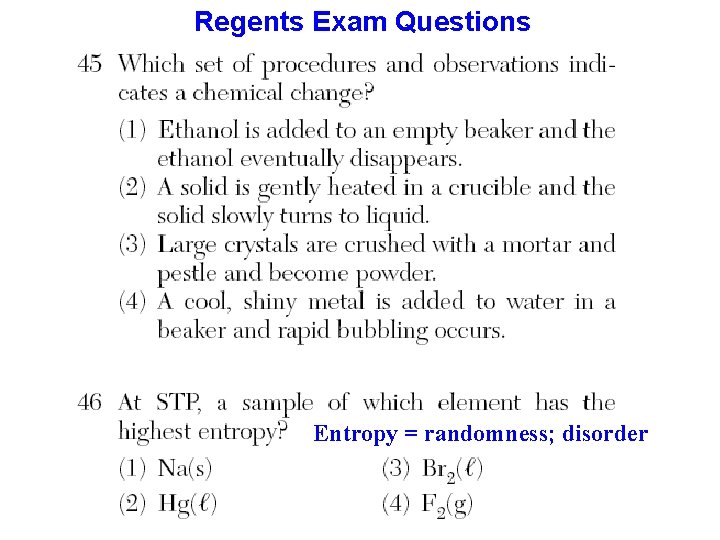
Regents Exam Questions Entropy = randomness; disorder
 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What is white matter made of
What is white matter made of Composition of matter section 1
Composition of matter section 1 Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Optic tract
Optic tract Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Frontal and parietal lobes
Frontal and parietal lobes Flow of energy vs flow of matter
Flow of energy vs flow of matter Definition of substance
Definition of substance Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 study guide the mole
Chapter 10 study guide the mole Examples of matter in chemistry
Examples of matter in chemistry Flowchart for matter
Flowchart for matter 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 2 matter and change answer key
2 matter and change answer key Matter flowchart chemistry
Matter flowchart chemistry Classification of matter graphic organizer
Classification of matter graphic organizer Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter Properties of liquids
Properties of liquids Properties of matter vocabulary
Properties of matter vocabulary Classification of matter concept map
Classification of matter concept map Objectives of properties of matter
Objectives of properties of matter General properties of matter
General properties of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Measurable properties examples
Measurable properties examples