Physical Change Phases of Matter Waters Phases Water






- Slides: 10

Physical Change Phases of Matter

Waters Phases: “Water” “Ice” “Vapor” They might seem completely different… But are they really? ***You learned about the details of phases themselves in 7 th grade. We are going to focus on WHY and HOW they change.

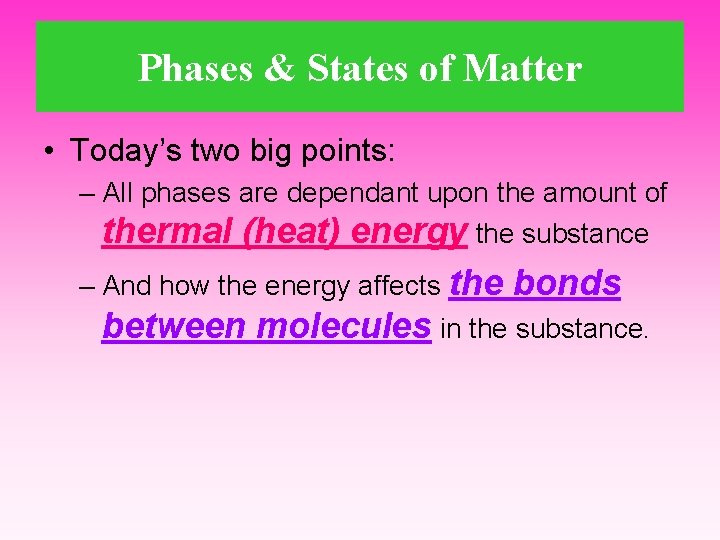
Phases & States of Matter • Today’s two big points: – All phases are dependant upon the amount of thermal (heat) energy the substance – And how the energy affects the bonds between molecules in the substance.

Property you need to know: If a substance is a solid, liquid, or gas at a certain temperature: That is a Property of that substance! EX: Water is a good example since it is so simple! <0 degrees Celsius = Solid (if you stuck a thermometer in a solid piece of “ice” what would be a temperature you would expect? ) >0 degrees Celsius = Liquid (if you stuck a thermometer in a glass of liquid water what would be a temperature you would expect? ) >100 degrees Celsius = Gas Water is pretty much the ONLY substance that changes phase at these temperatures. For example: Nitrogen will still be a gas at -196 degrees Celsius!!! Nitrogen will MELT at -210 degrees Celsius (-346 F)!!! ***All substances change phase at a certain temperature unique for that substance. “Hot” and “cold” don’t matter…

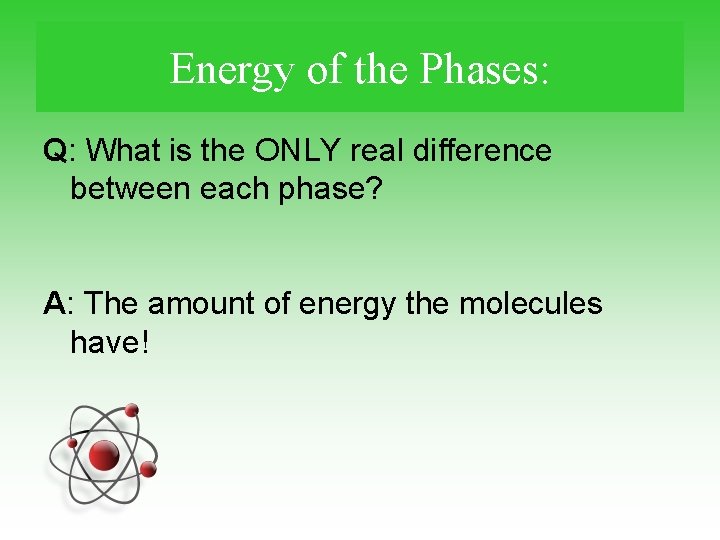
Energy of the Phases: Q: What is the ONLY real difference between each phase? A: The amount of energy the molecules have!

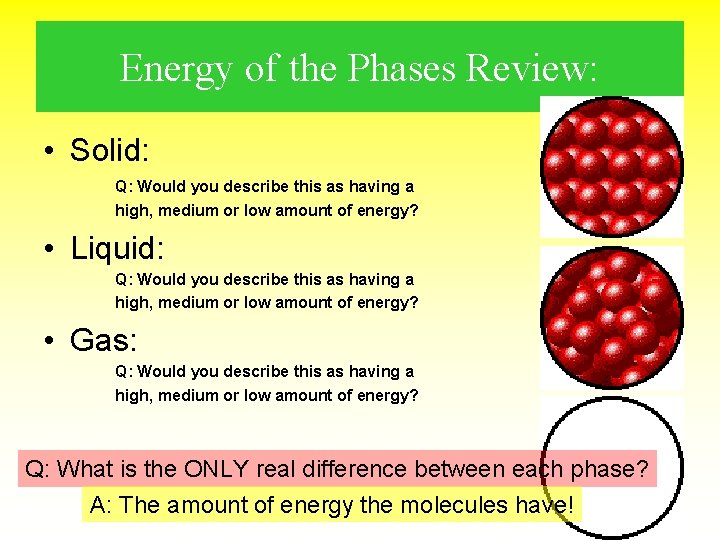
Solids • Very little energy – Not enough energy to break the bonds between molecules • Strong Bonds – Definite Shape (doesn’t change) – Definite Volume (can measure it) • Molecules very close together This picture shows what the atoms in a solid are doing (if we could see them)

Liquids • “Medium” Energy – Some bonds get broken – but they keep reforming. • “Medium” Bond Strength – No Definite Shape (Takes shape of “container”) – Definite Volume (Can measure it) • Not as tightly packed together – Can move around some (“flow”) This picture shows what the atoms in a liquid are doing (if we could see them)

Gases • “High” amount of Energy – Enough energy to break the bonds • “Weak” Bond Strength – No Definite Shape (can’t see it) – No Definite Volume (difficult to measure) • Very spread out – Moving freely through container This picture shows what the atoms in a gas are doing (if we could see them)

Energy of the Phases Review: • Solid: Q: Would you describe this as having a high, medium or low amount of energy? • Liquid: Q: Would you describe this as having a high, medium or low amount of energy? • Gas: Q: Would you describe this as having a high, medium or low amount of energy? Q: What is the ONLY real difference between each phase? A: The amount of energy the molecules have!

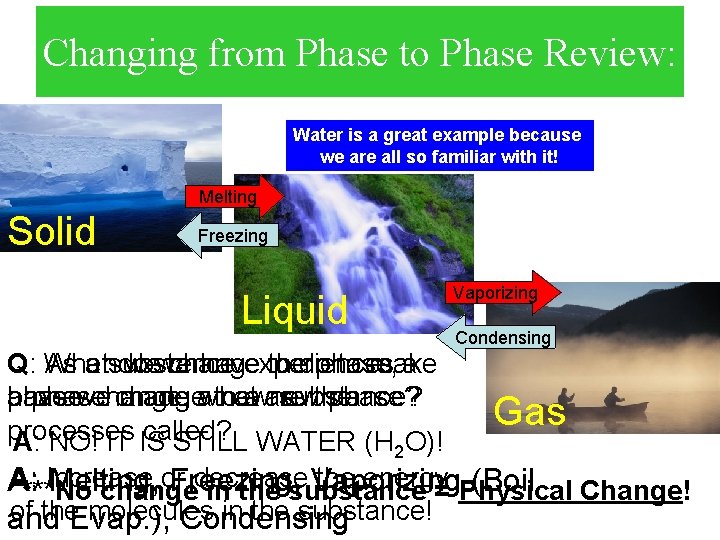
Changing from Phase to Phase Review: Water is a great example because we are all so familiar with it! Melting Solid Freezing Vaporizing Liquid Q: When What As a substance do wewe change have experiences to thedo phase, to make a a phase have we change, change made awhat to new a are new substance? the phase? processes called? A: NO! IT IS STILL WATER (H O)! 2 Condensing Gas A: Increase or decrease the energy A: Melting, Freezing, Vaporizing (Boil Change! ***No change in the substance = Physical of the. Evap. ), molecules in the substance! and Condensing
 Water and water and water water
Water and water and water water This water sport is done on rough river waters. *
This water sport is done on rough river waters. * Is mashing potatoes a physical or chemical change
Is mashing potatoes a physical or chemical change What is a physical change
What is a physical change Difference between physical change and chemical change
Difference between physical change and chemical change What is an example of chemical and physical change
What is an example of chemical and physical change Spare change physical versus chemical change
Spare change physical versus chemical change Physical change vs chemical change venn diagram
Physical change vs chemical change venn diagram How does a physical change differ from a chemical change
How does a physical change differ from a chemical change Chemical change baking
Chemical change baking Is chopping wood physical or chemical change
Is chopping wood physical or chemical change