Phases of Matter Phases Review Solid Definite shape







- Slides: 16

Phases of Matter

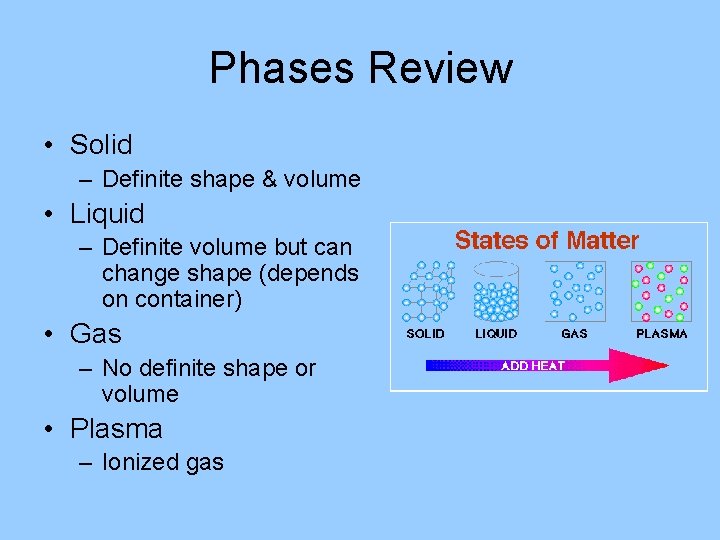
Phases Review • Solid – Definite shape & volume • Liquid – Definite volume but can change shape (depends on container) • Gas – No definite shape or volume • Plasma – Ionized gas

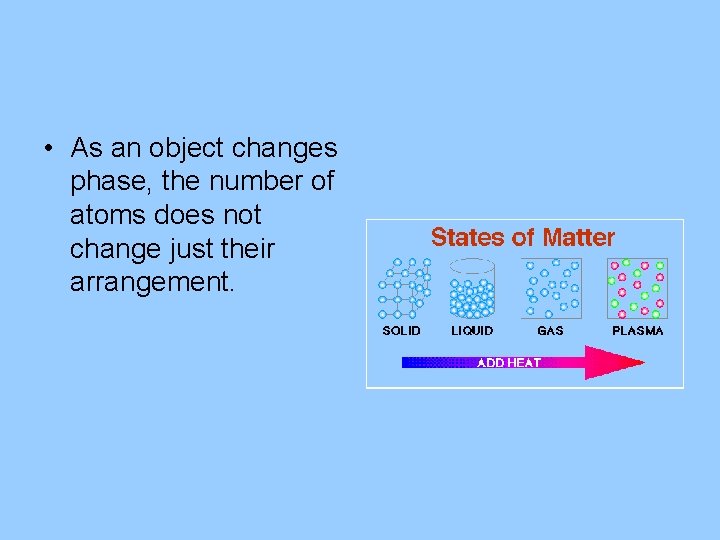
• As an object changes phase, the number of atoms does not change just their arrangement.

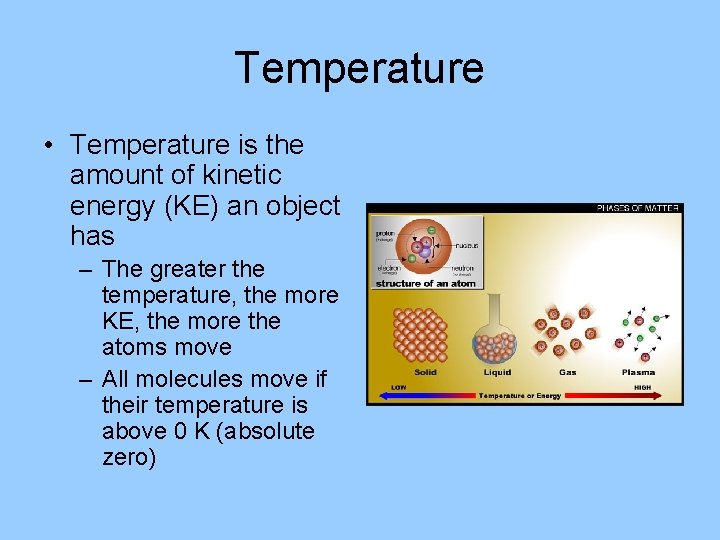
Temperature • Temperature is the amount of kinetic energy (KE) an object has – The greater the temperature, the more KE, the more the atoms move – All molecules move if their temperature is above 0 K (absolute zero)

• Objects at the same temperature have the same KE • even though objects have different properties they have same KE at the same temperature.

• If all molecules have the same kinetic energy at the same temperature, why don’t all things have the same melting and boiling points? – Different molecules have different intermolecular forces acting on them • Stronger the intermolecular force, the higher the melting and boiling points

• The more KE an object has, the faster the molecules will move (creating disorder). • Entropy = disorder • As temperature increases, entropy increases. • Amount of entropy Solid < liquid < gas < plasma


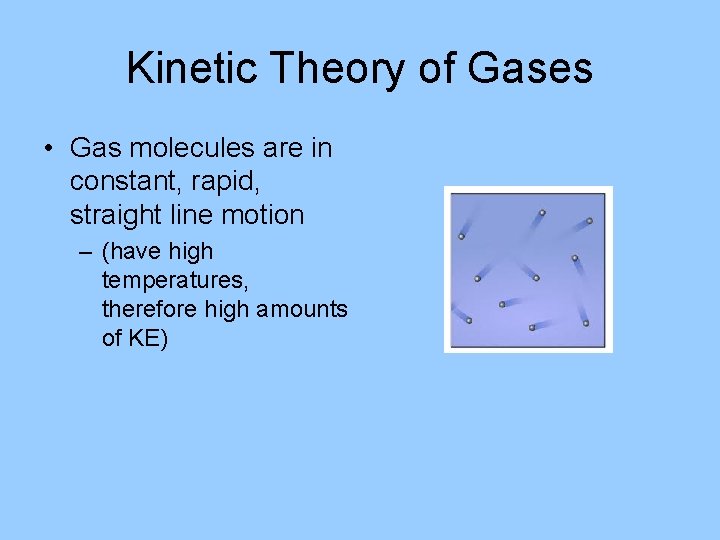
Kinetic Theory of Gases • Gas molecules are in constant, rapid, straight line motion – (have high temperatures, therefore high amounts of KE)

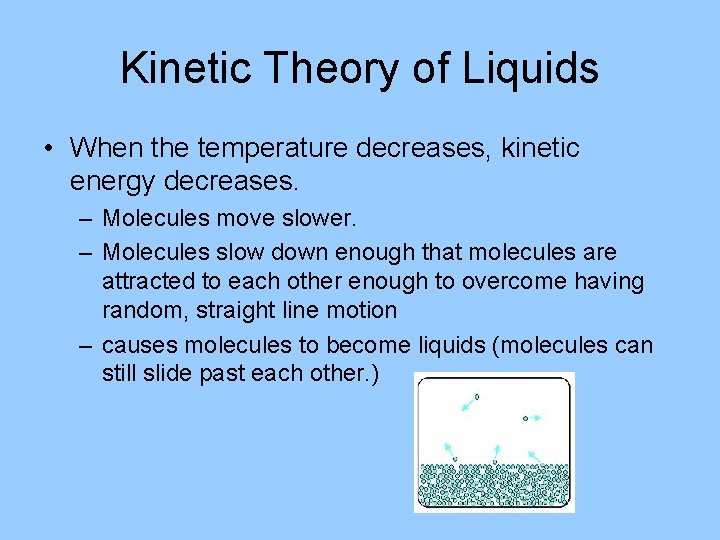
Kinetic Theory of Liquids • When the temperature decreases, kinetic energy decreases. – Molecules move slower. – Molecules slow down enough that molecules are attracted to each other enough to overcome having random, straight line motion – causes molecules to become liquids (molecules can still slide past each other. )

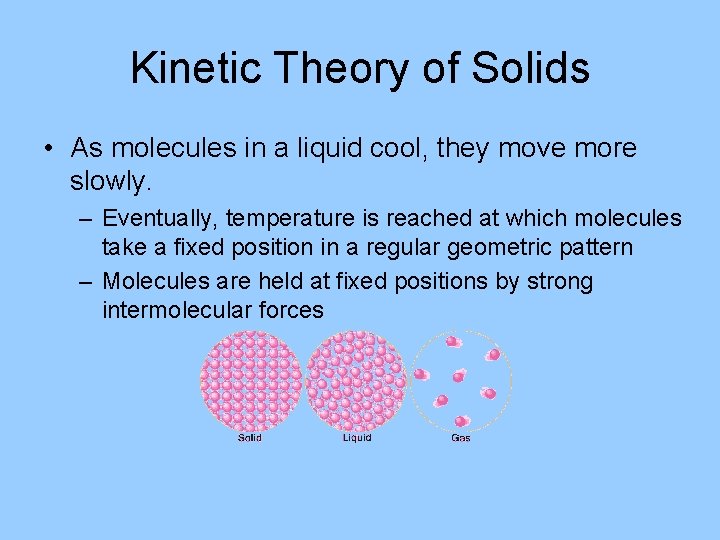
Kinetic Theory of Solids • As molecules in a liquid cool, they move more slowly. – Eventually, temperature is reached at which molecules take a fixed position in a regular geometric pattern – Molecules are held at fixed positions by strong intermolecular forces

Melting • As temperature increase, molecules vibrate faster and farther apart until the strong intermolecular forces are overcome enough for the molecules to glide past each other • Melting = endothermic.

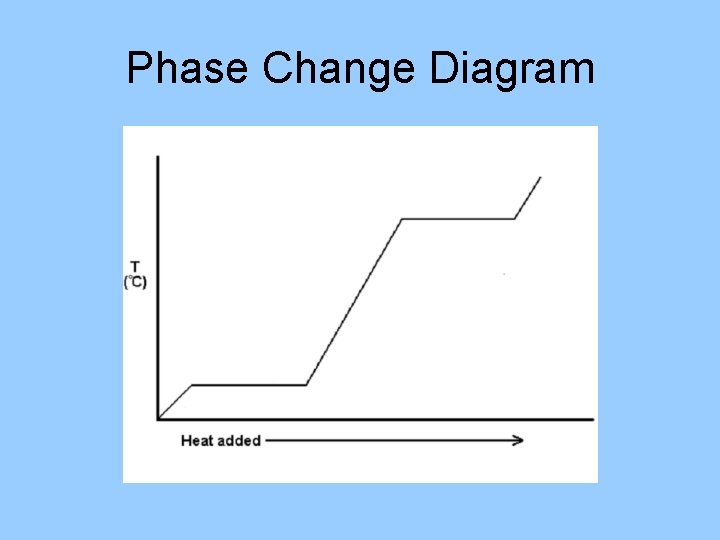
Changes of Phase terms • • • Freezing: liquid solid Melting: solid liquid Condensation: gas liquid Evaporation: liquid gas Sublimation: solid gas Deposition: gas solid

Phase Change Diagram


Solutions • Solutes impact boiling and melting points – Boiling point is raised when solutes are present • Presence of solutes makes it difficult for water to become a gas – Freezing point is depressed (lowered) • When solute is present in solvent, harder for solvent molecules to make crystal like structure